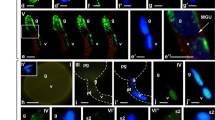
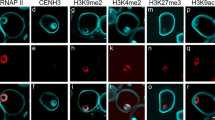
Abstract
We have analysed the distribution of epigenetic marks for histone modifications at lysine residues H3 and H4, and DNA methylation, in the nuclei of mature pollen cells of the Angiosperm tree Quercus suber; a monoecious wind pollinated species with a protandrous system, and a long post-pollination period. The ultrasonic treatment developed for the isolation of pollen nuclei proved to be a fast and reliable method, preventing the interference of cell wall autofluorescence in the in situ immunolabelling assays. In contrast with previous studies on herbaceous species with short progamic phases, our results are consistent with a high level of silent (5-mC and H3K9me2) epigenetic marks on chromatin of the generative nucleus, and the prevalence of active marks (H3K9me3 and H4Kac) in the vegetative nucleus. The findings are discussed in terms of the pollination/fertilization timing strategy adopted by this plant species.



Similar content being viewed by others
References
Bennett M, Leitch I (2004) Angiosperm DNA c-values database, release 5.0, Dec 2004. http://www.kew.org/cval/homepage.html
Berger SL (2007) The complex language of chromatin regulation during transcription. Nature 447:407–412
Bino RJ, Tuyl JM, De Vries JN (1990) Flow cytometric determination of relative nuclear DNA contents in bicellulate and tricellulate pollen. Ann Bot 65:3–8
Boavida LC, Varela MC, Feijó JA (1999) Sexual reproduction in the cork oak (Quercus suber L.). I. The progamic phase. Sex Plant Reprod 11:347–353
Castilho A, Neves N, Rufini-Castiglione M, Viegas W, Heslop-Harrison JS (1999) 5-Methylcytosine distribution and genome organization in Triticale before and after treatment with 5-azacytidine. J Cell Sci 112:4397–4404
Craiq JM (2005) Heterochromatin-many flavours, common themes. Bioessays 27:17–28
Fuchs J, Demidov D, Houben A, Schubert I (2006) Chromosomal histone modification patterns––from conservation to diversity. Trends Plant Sci 11:199–208
Horn PJ, Peterson CL (2006) Heterochromatin assembly: a new twist on an old model. Chromosome Res 14:83–94
Houben A, Demidov D, Gernand D, Meister A, Leach CR, Schubert I (2003) Methylation of histone H3 in euchromatin of plant chromosomes depends on basic nuclear DNA content. Plant J 33:967–973
Janousek B, Zluvova J, Vyskot B (2000) Histone H4 acetylation and DNA methylation dynamics during pollen development. Protoplasma 211:116–122
Janousek B, Matsunaga S, Kejnovsky E, Zluvova J, Vyskot B (2002) DNA methylation analysis of a male reproductive organ specific gene (MROS1) during pollen development. Genome 45:930–937
Jasencakova Z, Meister A, Walter J, Turner BM, Schubert I (2000) Histone H4 acetylation of euchromatin and heterochromatin is cell cycle dependent and correlated with replication rather than with transcription. Plant Cell 12:2087–2100
Jasencakova Z, Meister A, Schubert I (2001) Chromatin organization and its relation to replication and histone acetylation during the cell cycle in barley. Chromosoma 110:83–92
Jasencakova Z, Soppe WJ, Turner BM, Schubert I (2003) Histone modifications in Arabidopsis-high methylation of H3 lysine 9 is dispensable for constitutive heterochromatin. Plant J 33:471–480
Loureiro J, Rodriguez E, Dolezel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot 100:875–888
Oakeley J, Podesta A, Jost JP (1997) Developmental changes in DNA methylation of the two tobacco pollen nuclei during maturation. Proc Natl Acad Sci 94:11721–11725
Okada T, Singh MB, Bhalla PL (2006) Histone H3 variants in male gametic cells of lily and H3 methylation in mature pollen. Plant Mol Biol 62:503–512
Okada T, Singh MB, Bhalla PL (2007) Transcriptome profiling of Lilium longiflorum generative cells by cDNA microarray. Plant Cell Rep 26:1045–1052
Pan G, Zhou Y, Fowke LC, Wang H (2004) An efficient method for flow cytometric analysis of pollen and detection of 2n nuclei in Brassica napus pollen. Plant Cell Rep 23:196–202
Richards EJ, Elgin SC (2002) Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108:489–500
Schwarzacher T, Heslop-Harrison P (2000) Practical in situ hybridization. BIOS Scientific Publishers, Oxford, p 203
Tanaka I (1997) Differentiation of generative and vegetative cells in angiosperm pollen. Sex Plant Reprod 10:1–7
Weterings K, Russell SD (2004) Experimental analysis of the fertilization process. Plant Cell 16:S107–S118
Acknowledgments
We thank Dr. Andreas Houben (Leibniz Institute of Plant Genetics and Crop Plant Research) for kindly providing antibody against H3K9Ac. We are grateful to Augusta Barão for excellent technical assistance and Xana Castilho for her involvement with the manuscript. We also wish to thank Prof Neil Jones for his critical revision of the manuscript and editing of English. TR was supported by Fundação para a Ciência e Tecnologia with grant SFRH/BD/13319/2003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Dresselhaus.
Rights and permissions
About this article
Cite this article
Ribeiro, T., Viegas, W. & Morais-Cecílio, L. Epigenetic marks in the mature pollen of Quercus suber L. (Fagaceae). Sex Plant Reprod 22, 1–7 (2009). https://doi.org/10.1007/s00497-008-0083-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-008-0083-y