Summary
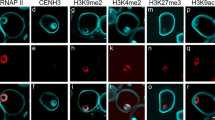
The first pollen mitosis results in generative and vegetative cells which are characterised by a striking difference in their chromatin structure. In this study, histone H4 acetylation and DNA methylation have been analysed during pollen development inLilium longiflorum. Indirect immunofluorescence procedures followed by epifluorescence and laser scanning microscopy enabled a relative quantification of H4 acetylation and DNA methylation in microspores, immature binucleate pollen, mature pollen, and pollen tubes. The results show that histone H4 of the vegetative nucleus, in spite of its decondensed chromatin structure, is strongly hypoacetylated at lysine positions 5 and 8 in comparison with both the original microspore nucleus and the generative-cell nucleus. These H4 terminal lysines in the vegetative nucleus are, however, progressively acetylated during the following pollen tube growth. The DNA methylation analysis inversely correlates with the histone acetylation data. The vegetative nucleus in mature pollen grains is heavily methylated, but a dramatic nonreplicative demethylation occurs during the pollen tube development. Changes neither in H4 acetylation nor in DNA methylation have been found during development of the generative nucleus. The results obtained indicate that the vegetative nucleus enters the quiescent state (accompanied by DNA hypermethylation and H4 underacetylation) during the maturation of pollen grain which enables pollen grains a long-term survival without external source of nutrients until they reach the stigma.
Similar content being viewed by others
References
Adams RLP (1996) DNA methylation. Principles Med Biol 5: 33–66
Barnabas B (1985) Effect of water loss on germination ability of maize (Zea mays) pollen. Ann Bot 48: 861–864
Bhattacharya SK, Ramchandani S, Cervoni N, Szyf M (1999) A mammalian protein with specific demethylase activity for mCpG DNA. Nature 379: 579–583
Buzek J, Riha K, Siroky J, Ebert I, Greilhuber J, Vyskot B (1998) Histone H4 underacetylation in plant facultative heterochromatin. Biol Chem 379: 1235–1241
Collas P (1998) Modulation of plasmid DNA methylation and expression in zebrafish embryos. Nucleic Acids Res 26: 4454–4461
Eady C, Lindsey K, Twell D (1995) The significance of microspore division and division symmetry for vegetative cell-specific transcription and generative cell differentiation. Plant Cell 7: 65–74
Hladilova R, Siroky J, Vyskot B (1998) A cytospin technique for spreading plant metaphases suitable for immunofluorescence studies. Biotech Histochem 73: 150–156
Houben A, Belyaev ND, Turner BM, Schubert I (1996) Differential immunostaining of plant chromosomes by antibodies recognising acetylated histone H4 variants. Chromosome Res 4: 191–194
— —, Leach CR, Timmis JN (1997) Differences of histone H4 acetylation and replication timing between A and B chromosomes ofBrachycome dichromosomatica. Chromosome Res 5: 233–237
Iwanami Y (1984) The viability of pollen grains of lily (Lilium auratum) and the eggs of the brine shrimp (Artemia salina) soaked in organic solvents for 10 years. Experientia 40: 568–569
Jeppesen P, Turner BM (1993) The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell 74: 281–289
Kass SU, Pruss D, Wolffe AP (1997) How does DNA methylation repress transcription? Trends Genet 13: 444–449
La Fountain KL, Mascarenhas JP (1972) Isolation of vegetative and generative nuclei from pollen tubes. Exp Cell Res 73: 472–476
Mascarenhas JP (1993) Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5: 1303–1314
—, Bell E (1970) RNA synthesis during development of the male gametophyte ofTradescantia. Dev Biol 21: 475–490
Miller DD, Scordilis SP, Kepler PK (1995) Identification and localisation of three classes of myosins in pollen tubes of theLilium longiflorum andNicotiana alata. J Cell Sci 108: 2543–2653
Oakeley EJ, Podesta A, Jost J-P (1997) Developmental changes in DNA methylation of the two tobacco pollen nuclei during maturation. Proc Natl Acad Sci USA 94: 11721–11725
Podesta A, Ruffini Castiglione M, Avanzi S, Montagnoli G (1993) Molecular geometry of antigen binding by a monoclonal antibody against 5-methylcytidine. Int J Biochem 25: 929–933
Razin A, Cedar H (1993) DNA methylation and embryogenesis. In: Jost JP, Saluz HP (ed) DNA methylation: molecular biology and molecular significance, Birkhäuser, Basel, pp 343–357
Suzuki T, Ide N, Tanaka I (1997) Immunocytochemical visualisation of the centromeres during male and female meiosis inLilium longiflorum. Chromosoma 106: 435–445
Tanaka I, Ono K, Fukuda T (1998) The developmental fate of angiosperm pollen is associated with a preferential decrease in the level of histone H1 in the vegetative nucleus. Planta 206: 561–569
Turner BM (1998) Histone acetylation as an epigenetic determinant of the long-term transcriptional competence. Cell Mol Life Sci 54: 21–31
—, Fellows G (1989) Specific antibodies reveal ordered and cell-cycle-related use of histone-H4 acetylation sites in mammalian cells. Eur J Biochem 179: 131–139
Ueda K, Tanaka I (1995) The appearance of male-gametic nucleus gH2B and gH3 histones during pollen development inLilium longiflorum. Dev Biol 169: 210–217
Ura K, Kurumizaka H, Dimitrov S, Almouzni G, Wolffe AP (1997) Histone acetylation: influence on transcription, nucleosome mobility and positioning, and linker-histone dependent transcriptional repression. EMBO J 16: 2096–2107
Vyskot B (1999) The role of DNA methylation in plant reproductive development. In: Ainsworth CC (ed) Sex determination in plants. Bios Scientific Publishers, Oxford, pp 101–120
Wolffe AP, Khochbin S, Dimitrov S (1997) What do linker histones in chromatin. BioEssays 19: 249–255
Zhang H-Q, Bohdanowicz J, Pierson S-E, Li Y-Q, Tiezzi A, Cresti M (1995) Microtubular organization during asymmetrical division of the generative cell inGagea lutea. J Plant Res 108: 269–276
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Janousek, B., Zluvova, J. & Vyskot, B. Histone H4 acetylation and DNA methylation dynamics during pollen development. Protoplasma 211, 116–122 (2000). https://doi.org/10.1007/BF01279904
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01279904