Abstract
Febrile seizures are convulsions predominately occurring in young children. The effects of various exposomes, including influenza infection and external environmental factors, on febrile seizures have not been well-studied. In this study, we elucidated the relationships between ambient temperature, air pollutants, influenza infection, and febrile seizures using 22-year territory-wide hospitalization data in Hong Kong. The aggregated data were matched with the meteorological records and air pollutant concentrations. All-type and type-specific influenza-like illness positive (ILI+) rates were used as proxies for influenza activity. Distributed lag non-linear model in conjunction with the quasi-poisson generalized additive model was used to examine the associations of interest. According to the results, all-type influenza infections were significantly associated with an increased risk of hospital admissions for febrile seizures (cumulative adjusted relative risk [ARR] = 1.59 at 95th percentile vs. 0; 95% CI, 1.51–1.68). The effect of ILI + A/H3N2 on febrile seizure was more pronounced than other type-specific ILI + rates. A low mean ambient temperature was identified as a significant risk factor for febrile seizures (cumulative ARR = 1.50 at 5th percentile vs. median; 95% CI, 1.35–1.66), while the redox-weighted oxidant capacity and sulfur dioxide were not associated with febrile seizures. In conclusion, our study underscores that influenza infections and exposure to cold conditions were related to an increased risk of febrile seizures in children. Thus, we advocate for influenza vaccination before the onset of the cold season for children to mitigate the burden of febrile seizures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Febrile seizures, also known as febrile convulsions or fever fits, refers to convulsions that is associated with fevers but without the presence of intracranial infection, hypoglycemia, or an acute electrolyte imbalance (Sadleir and Scheffer 2007). Febrile seizures mainly occur in infants and children aged six months to six years, affecting 2–5% of those under five, with even higher rates observed in developing settings (Verity et al. 1985). The majority of these incidents are seen in children under the age of 3 years, primarily because their brains are more susceptible due to immaturity (Sharawat et al. 2016). Symptoms of febrile seizure manifestation typically include fever, lose consciousness, irregular breathing, arms and legs twitching, eyes rolling upwards (Paul et al. 2015). While febrile seizures are generally benign and not indicative of serious health issues in children (Nelson and Ellenberg 1976), they can give rise to unease or even extreme fear for parents (Baumer et al. 1981).
Febrile seizures are triggered by fever, which can result from any illness that elevates body temperature. Among these, viral infection stands as a prevalent cause of fever leading to febrile seizures (Smith et al. 2019). An observational study by Francis et al. (2016) highlighted that 71% of the febrile seizure cases were associated with viral infections, while Lewis et al. (1979) reported an even higher correlation, with 86% of cases being virus-related. Specifically, influenza was recognized as a significant viral contributor to febrile seizures. A five-year retrospective study (Chung and Wong 2007) showed that among five common viruses that infect human beings, including influenza, adenovirus, parainfluenza, respiratory syncytial virus and rotavirus, influenza emerged as the most frequently associated virus (17.6%) in febrile seizure incidence among children. Moreover, this study noted that 20.8% of children hospitalized with influenza exhibited febrile seizures, a higher relative risk compared to other infections. Supporting this, a retrospective cohort study conducted by Chiu et al. (2001) found that influenza A was implicated in 19.5% of febrile seizure cases, a rate significantly higher than whose associated with parainfluenza and adenovirus.
Recent findings have highlighted the environmental exposome, including air pollutants and meteorological factors, as significant risk factors for febrile seizures. Christensen et al. (2022) observed a notably higher occurrence of febrile seizure during winter compared to the summer, echoing another study conducted in South Korea by Kin et al.(Kim et al. 2017) Analyses from two time-series studies indicated that lower ambient mean temperature, higher O3 concentration and lower NO2 concentration were statistically significantly associated with hospital visit due to febrile seizure (Kim et al. 2017, 2019). However, the findings were inconsistent with the other investigations (Hjortebjerg et al. 2018; Kawakami et al. 2020), indicating a need for further research to fully understand the environmental influences on febrile seizure risk.
To the best of our knowledge, only one study conducted in Japan (Kawakami et al. 2020) has quantified the impact of multiple exposome, including influenza infection and environmental factors on febrile seizure simultaneously, though the potential lagged effect of the factors has not been accounted for in this research (Kim et al. 2017; Chai et al. 2020). In this study, we aim to elucidate the relationship between cold conditions, air pollutants, influenza infections, and febrile seizure manifestations, leveraging comprehensive population-based data in Hong Kong.
Materials and methods
Data source
The study period is from June 1998, to December 2019. Weekly hospital admissions within the public healthcare sector, diagnosed with febrile seizure (International Classification of Diseases ICD9: 780.31) and weekly all-cause hospital admissions were obtained from the surveillance data provided by the Hong Kong Hospital Authority. Children aged 0 to 3 years old who had been diagnosed with febrile seizure were included in the study. Weekly consultation rates for influenza-like illness (ILI), defined as a fever of greater than or equal to 38.5oC, in addition to cough and sore throat, were collected from the Centre for Health Protection, Hong Kong (Cowling et al. 2006). Weekly influenza activity was approximated by influenza-like illness positive (ILI+) rate in this study (Chong et al. 2022; Li et al. 2023; Xiong et al. 2023). ILI + rate was derived by multiplying the proportion of community ILI consultations and the proportion of positive respiratory specimens for a particular influenza subtype, utilizing the surveillance data from the Centre for Health Protection, Hong Kong. The weekly combined rate of all influenza strains (i.e., ILI + total) and rates for specific influenza strains, including ILI + A/H1N1, A/H3N2 and B, were derived for this analysis. Weekly mean ambient temperature data were obtained from the Hong Kong Observatory. Weekly air pollutant data, including the mean concentration of NO2, SO2, O3 and PM2.5, were derived by averaging the data collected from over 10 monitoring stations of the Environmental Protection Department in Hong Kong. To ensure the alignment of data, all datasets, including ILI and environmental variables, were consistently compiled on a weekly basis, from Sunday to Saturday.
Statistical analysis
Previous studies have demonstrated that both influenza and environmental factors could exert delayed and non-linear effects on health outcomes (Kim et al. 2019; Mohammad et al. 2021). In order to examine the associations of interest while accounting for the abovementioned effects, we employed a quasi-poisson generalized additive model in combination with the distributed lag non-linear model (DLNM) (Gasparrini et al. 2010). The weekly number of hospital admissions due to febrile seizures was set as the outcome variable. The model used for the analysis is as follows:
where µt is the expected number of hospital admissions for febrile seizure in week t. cb(.) represents a cross-basis function that models the common exposure-response relationship and lagged effects of explanatory variables at once (Gasparrini et al. 2011). s(.) denotes a smoothing spline function. ILI + t represents the rate of ILI + in week t. Mean temperature (tempt) in week t was included to explore the meteorological effects on the outcome. The weekly mean concentration of sulfur dioxide (SO2t) and redox-weighted oxidant capacity (Oxt) were involved to explore the effects of air pollutants. Ox represents the combined oxidant capacity of O3 and NO2, and it was employed to avoid colinearity between O3 and NO2. Ox is the weighted average of O3 and NO2 by their respective redox potentials (i.e. Ox = (1/3)NO2+ (2/3)O3) (Weichenthal et al. 2017), and it has been demonstrated as a better indicator of atmospheric oxidative capacity than unweighted oxidant capacity (Guo et al. 2018). The effect of PM2.5 was examined separately from Ox and SO2 to avoid a colinearity. yeart and weekt denote year and week of the year, and they were included to account for the long-term trends and seasonality, respectively. offsett is the offset term of this regression model, which is the natural logarithm of the number of all-cause hospital admissions of children aged 0 to 3 in week t. It serves to adjust our analysis for differences in the population at risk each week, allowing the comparison of febrile seizure rates across different covariates. The degrees of freedom of the exposure variables and the lag effect were chosen in a range of two to five and optimized by minimizing the generalized cross-validation score. The maximum lag time was set to 2 weeks following the similar studies (Chong et al. 2022; Mohammad et al. 2021). The impact of influenza and environmental exposures on febrile seizure admissions was quantified by cumulative adjusted relative risk (ARR), which accumulates the relative risk over the lag period, along with the corresponding 95% confidence intervals (CI). An ARR with 95% CI excluding one indicates statistically significance. The reference level of ILI + rates was set as zero. The reference values for ambient temperature were set to its median, and the reference values for air pollutants were set to their respective 5th percentiles (Leung et al. 2021; Mohammad et al. 2021). Adjusted R-square was used to evaluate the goodness of fit of our models, with higher values indicating a better fit.
Sensitivity analysis
Given the unique health landscape during the 2009 influenza pandemic in Hong Kong, which potentially altered the seasonality of hospital admission trends and could affect the robustness and generalizability of our study outcomes, we conducted a sensitivity analysis by excluding the data from 2009. This exclusion aimed to evaluate the pandemic’s impact on the observed magnitude of febrile seizures in relation to total and type-specific ILI + rates and environmental factors. Further, with the Environmental Protection Department of the government of Hong Kong launching the Air Quality Health Index (AQHI) on December 30, 2013, to provide the public with real-time air pollutant data with relevant health advice (Environmental Protection and Department 2013), we hypothesized that public behavior and exposure to environmental pollutants might have changed significantly thereafter. Hence, a second sensitivity analysis was conducted by excluding the data collected after week 52, 2013 to assess the potential influence of these changes on our study outcomes. For both sensitivity analyses, we replicated the initial statistical procedures on the adjusted datasets to compare the outcomes with and without the excluded periods. Differences in results between the initial analysis and each sensitivity analysis were carefully examined to assess the impact of these exclusions on our findings. This comparative approach was designed to specifically ascertain the stability of the relationship between ILI+, environmental factors, and the incidence of febrile seizures under varying conditions.
All statistical analyses were conducted using the “dlnm” and “mgcv” packages in the R environment (version 4.1.2).
Ethics approval and consent to participate
Ethics approval and consent to participate are not required as only retrospective aggregated data were used.
Results
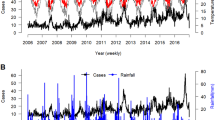
From June 1998 to December 2019, we recorded a total of 41,407 hospital admissions for febrile seizures among children aged 0 to 3 years. The seasonality of febrile seizure rate is depicted in Fig. 1. The weekly median (inter-quartile range) rate of febrile seizures was 18 (14–24) per 1000 consultations, and the weekly median rates of ILI + total, ILI + A/H1N1, ILI + A/H3N2, and ILI + B were 3.32 (1.19–7.76), 0.22 (0.01–1.11), 0.93 (0.26–3.31), and 0.47 (0.15–1.43) per 1000 consultations respectively (Table 1). In 2009, Hong Kong experienced an aberrant upsurge in ILI + A/H1N1 rate (Fig. 2). The weekly median levels of temperature, was 24.6oC (19.5oC -28.0oC). The weekly O3 level was 37.1 µg/m3 (26.2 µg/m3-49.4 µg/m3), and the weekly SO2 and NO2 levels were13.3 µg/m3 (9.2 µg/m3-18.7 µg/m3) and 51.5 µg/m3 (41.5 µg/m3-60.5 µg/m3), respectively. The weekly PM2.5 level was 30.3 µg/m3 (20.6 µg/m3-42.8 µg/m3). Kendall’s Tau correlation coefficients between environmental variables are shown in Table S1 in the Appendix. NO2, O3 and PM2.5 have moderate to strong correlations with one another. The adjusted R-square for the models ranged from 0.524 to 0.668 (Table S2, Appendix), indicating moderate to good fitting of the models.
The effects of ILI + total and type-specific ILI + on febrile seizures are shown in Fig. 2 while the lag-specific effects are presented in Table 2. The cumulative ARR of febrile seizures increased to 1.59 (95% CI, 1.51–1.68) when the ILI + total rate reached the 95th percentile. Similar monotonic increasing trends of the risks were observed for type-specific ILI + rates, with the effect of ILI + A/H3N2 on febrile seizure being more pronounced. The cumulative ARR of febrile seizure rose to 1.38 (95% CI, 1.31–1.45) when the ILI + ILI + A/H3N2 rate was at the 95th percentile. The most substantial risk for febrile seizure admissions due to all ILI + was identified at week zero, with all risks remaining significantly above 1 at a one-week lag.
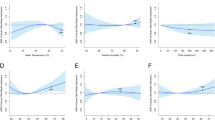
A low ambient temperature was statistically significantly associated with a higher risk of febrile seizures (Fig. 3). Using the median temperature (24.6ºC) as the reference value, the cumulative ARRs of febrile seizure admissions at the 5th percentile (i.e., 15.1oC) was 1.50 (95% CI: 1.35–1.66). The cumulative ARRs of PM2.5 were statistically significantly below unity over the 5th to the 99th concentration level, with the cumulative ARRs at the 95th percentile (65.3 µg/m3) being 0.80 (95% CI: 0.72–0.89). The cumulative ARRs for Ox and SO2 were not significant over the entire observed range.
Cumulative adjusted relative risks (ARRs) with 95% confidence interval on febrile seizure admissions for children aged 0 to 3 years old against ambient temperature and pollutant covariates. (A) Mean ambient temperature; (B): Redox-weighted oxidant capacity; (C) Sulfur dioxide; (D) Fine particulate matter.
In the sensitivity analysis, we meticulously analyzed the differences between the outcomes of the initial analysis and each subsequent sensitivity analysis. A comparative review of Figure S1 against Figs. 1 and 2 revealed a general consistency in the effects of both total and type-specific ILI+, as well as all environmental factors, on febrile seizures (Figure S1, Appendix). This indicates that the relationships identified in our primary analysis are largely unaffected by the exclusion of data from 2009. Similarly, our results also remained robust upon excluding data collected after the AQHI was introduced, underscoring the stability of our results across various conditions (Figure S2, Appendix).
Discussion
In this study, we elucidated the relationships between the external exposome (i.e., ambient temperature and air pollutants), the internal exposome (influenza infection), and febrile seizures using territory-wide hospitalization data from Hong Kong. We identified seasonal influenza as a risk factor for febrile seizures, consistent with previous observational studies (Chung and Wong 2007; Chiu et al. 2001) that highlighted the current understanding of the pathogenic mechanisms of febrile seizures (Mosili et al. 2020). Influenza infection can induce an inflammatory response and then elevate the levels of cytokines such as interleukin-1β, IL-6, and tumour necrosis factor-α (Gu et al. 2021; Han and Han 2023; Vezzani and Viviani 2015). The increase in these cytokines can subsequently raise core body temperature, further triggering convulsions. In Addition, we demonstrated that the associations between febrile seizures and influenza A/H1N1, A/H3N2 and B were all statistically significant at the population level. Among the influenza types, the effect of influenza A/H3N2 on febrile seizures was more pronounced, aligning with the findings of other observational studies. These studies reported that influenza A/H3N2 is associated with more severe clinical symptoms than influenza A/H1N1 and B, including a higher frequency of fever and higher body temperature (Kaji et al. 2003; Silvennoinen et al. 2015). Considering the safety and protective effect of pediatric influenza vaccination (France et al. 2004; Jefferson et al. 2018), we recommend seasonal vaccination for vulnerable children to protect them from influenza infection and febrile seizures.
Our study found that cold conditions were independently associated with a higher risk of febrile seizure admissions, regardless of influenza activity, being in line with previous research (Kim et al. 2017). We speculate that this association could be resulted from the abnormalities of thermoregulation. Exposure to cold ambient temperatures can trigger the hypothalamus to signal the skin, muscles and various organs to produce additional heat to maintain normal body temperature (Osilla et al. 2022). However, since the hypothalamus of infants and children is not fully developed, this process can lead to overproduction and fever. Additionally, some studies indicated that cold temperatures can could weaken the immune response (Hu et al. 2016; Kelley 1984), making people being more susceptible to fever-inducing agents. Along with climate change, it is expected that more extremely cold weathers could occur (Cohen et al. 2020, 2021), the susceptibility of children to febrile seizure is expected to increase.
Regarding the effect of air pollutants, our findings indicated that Ox and SO2 were not associated with febrile seizure manifestation. PM2.5 was found to be a protective factor for febrile seizures in our study. However, the association seemed biologically implausible, given that PM2.5 is generally considered harmful to human health, and exposure to PM2.5 has been found to induce in systemic inflammation in humans (Schneider et al. 2011; Zhao, 2013). It is possible that various air quality control measures and policies enacted during our study period may explain this unexpected result. Such initiatives likely reduced the concentration of PM2.5 (Lin et al. 2018), and may lead to behavioral changes among parents, such as limiting outdoor activities for their children, keeping windows closed to maintain clean indoor air, and using air purifiers. In this case, despite the high ambient levels of PM2.5, actual exposure may have been minimized. Nevertheless, to the best of our knowledge, no study has yet delineated the specific biological mechanism by which air pollutants influence febrile seizure or other convulsions, underscoring the need for further experimental investigation.
A major advantage of this study is the data completeness, a feature often missing in research from developing settings (Waruiru et al. 2004). Our analysis utilizes daily data spanning 22 years, emcompassing the majority of hospital admission cases in Hong Kong. Also, the clear and consistent timing and intervals of the data points in the surveillance data allowed for the effective application of DLNM model to account for the lag effects of the variables under study, thereby minimizing bias. Moreover, employing ILI + as a proxy of influenza cases addresses the common issue of under-reporting, enabling more accurate estimations (Mohammad et al. 2020). Given the current uncertainty surrounding the precise mechanisms of febrile seizures, the associations observed between environmental factors and febrile seizure in this study could also offer valuable insights into understanding the underlying biological mechanisms.
This study is subjected to several limitations. Firstly, ecological fallacy may be a problem since we used aggregated data to estimate the associations of interest. Secondly, individual risk factors for febrile seizure manifestation, such as age, genetic predisposition, vaccination and medical history (Chung 2014; Graves et al. 2012; Principi and Esposito 2013), were unable to be accounted for in this study. Thirdly, our study did not account for the effects of other pathogens known to induce febrile seizures, such as respiratory syncytial virus (Leung et al. 2021), due to the unavailability of relevant surveillance data.
Conclusion
Influenza infections and cold conditions were significant exposomes related to an increased risk of febrile seizures in children. In settings where inpatient resources for managing febrile seizures are limited, it is highly recommended that children of eligible age receive the influenza vaccination to mitigate the incidence of febrile seizures. Additionally, it is crucial to ensure young children are kept warm during cold weather, especially in light of the increase of extreme cold days along with climate change.
Data availability
The sharing of data is restricted by Hong Kong Hospital Authority. All code used for the analyses can be provided upon request.
References
Baumer JH, David TJ, Valentine SJ, Roberts JE, Hughes BR (1981) Many parents think their child is dying when having a first febrile convulsion. Dev Med Child Neurol 23(4):462–464
Chai G, He H, Su Y, Sha Y, Zong S (2020) Lag effect of air temperature on the incidence of respiratory diseases in Lanzhou, China. Int J Biometeorol 64(1):83–93
Chiu SS, Tse CY, Lau YL, Peiris M (2001) Influenza a infection is an important cause of febrile seizures. Pediatrics 108(4):E63
Chong KC, Chen Y, Chan EYY, Lau SYF, Lam HCY, Wang P, Goggins WB, Ran J, Zhao S, Mohammad KN, Wei Y (2022) Association of weather, air pollutants, and seasonal influenza with chronic obstructive pulmonary disease hospitalization risks. Environ Pollut 293:118480
Christensen KJ, Dreier JW, Skotte L, Feenstra B, Grove J, Børglum AD, Mitrovic M, Cotsapas C, Christensen J (2022) Seasonal variation and risk of febrile seizures: a danish nationwide cohort study. Neuroepidemiology 56(2):138–146 https://doi.org/10.1159/000522065
Chung S (2014) Febrile seizures. Korean J Pediatr 57(9):384–395
Chung B, Wong V (2007) Relationship between five common viruses and febrile seizure in children. Arch Dis Child 92(7):589–593
Cohen J, Zhang X, Francis J, Jung T, Kwok R, Overland J, Ballinger T, Bhatt U, Chen H, Coumou D (2020) Divergent consensuses on Arctic amplification influence on midlatitude severe winter weather. Nat Clim Change 10(1):20–29
Cohen J, Agel L, Barlow M, Garfinkel CI, White I (2021) Linking Arctic variability and change with extreme winter weather in the United States. Science 373(6559):1116–1121. https://doi.org/10.1126/science.abi9167
Cowling BJ, Wong IO, Ho LM, Riley S, Leung GM (2006) Methods for monitoring influenza surveillance data. Int J Epidemiol 35(5):1314–1321
Environmental Protection, Department (2013) EPD - about AQHI. http://www.aqhi.gov.hk/en/what-is-aqhi/about-aqhi. html
France EK, Glanz JM, Xu S, Davis RL, Black SB, Shinefield HR, Zangwill KM, Marcy SM, Mullooly JP, Jackson LA, Chen R (2004) Safety of the trivalent inactivated influenza vaccine among children: a population-based study. Arch Pediatr Adolesc Med 158(11):1031–1036
Francis JR, Richmond P, Robins C, Lindsay K, Levy A, Effler PV, Borland M, Blyth CC (2016) An observational study of febrile seizures: the importance of viral infection and immunization. BMC Pediatr 16(1):202
Gasparrini A (2011) Distributed lag Linear and Non-linear models in R: the Package dlnm. J Stat Softw 43(8):1–20
Gasparrini A, Armstrong B, Kenward MG (2010) Distributed lag non-linear models. Stat Med 29(21):2224–2234
Graves RC, Oehler K, Tingle LE (2012) Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician 85(2):149–153
Gu Y, Zuo X, Zhang S, Ouyang Z, Jiang S, Wang F, Wang G (2021) The mechanism behind Influenza Virus Cytokine Storm. Viruses 13(7). https://doi.org/10.3390/v13071362
Guo H (2018) Comparisons of combined oxidant capacity and redox-weighted oxidant capacity in their association with increasing levels of FeNO. Chemosphere 211:584–590
Han JY, Han SB (2023) Pathogenetic and etiologic considerations of febrile seizures. Clin Exp Pediatr 66(2):46–53. https://doi.org/10.3345/cep.2021.01039
Hjortebjerg D, Andersen AM, Ketzel M, Raaschou-Nielsen O, Sørensen M (2018) Exposure to traffic noise and air pollution and risk for febrile seizure: a cohort study. Scandinavian journal of work, environment & health
Hu GZ, Yang SJ, Hu WX, Wen Z, He D, Zeng LF, Xiang Q, Wu XM, Zhou WY, Zhu QX (2016) Effect of cold stress on immunity in rats. Exp Ther Med 11(1):33–42
Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V (2018) Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2(2):CD004879
Kaji M, Watanabe A, Aizawa H (2003) Differences in clinical features between influenza A H1N1, a H3N2, and B in adult patients. Respirology 8(2):231–233
Kawakami I, Inoue M, Adachi S, Koga H (2020) The weather condition and epidemics as triggers for febrile seizure: a single-center retrospective observational study. Epilepsy Behav 111:107306
Kelley KW (1984) Immune responses and plasma hormone concentrations in cold-exposed, xeranol-implanted calves. Am J Vet Res 45(12):2617–2621
Kim SH, Kim JS, Jin MH, Lee JH (2017) The effects of weather on pediatric seizure: a single-center retrospective study (2005–2015). Sci Total Environ 609:535–540
Kim SH, Shim HS, Kang SM, Park H, Jin MH, Lee JH (2019) Are there effects of lunar cycle on pediatric febrile seizure? A single-center retrospective study (2005–2018). Sci Total Environ 692:589–594
Leung SY, Lau SY, Kwok KL, Mohammad KN, Chan PK, Chong KC (2021) Short-term association among meteorological variation, outdoor air pollution and acute bronchiolitis in children in a subtropical setting. Thorax 76(4):360–369
Lewis HM, Parry JV, Parry RP, Davies HA, Sanderson PJ, Tyrrell DA, Valman HB (1979) Role of viruses in febrile convulsions. Arch Dis Child 54(11):869–876
Li C, Jiang X, Yue Q, Wei Y, Wang Y, Ho JY, Lao XQ, Chong KC (2023) Relationship between meteorological variations, seasonal influenza, and hip fractures in the elderly: a modelling investigation using 22-year data. Sci Total Environ 862:160764
Lin C, Li Y, Lau AKH, Li C, Fung JCH (2018) 15-Year PM2.5 trends in the Pearl River Delta Region and Hong Kong from Satellite Observation. Aerosol Air Qual Res 18:2355–2362
Mohammad KN, Chan EY, Wong MC, Goggins WB, Chong KC (2020) Ambient temperature, seasonal influenza and risk of cardiovascular disease in a subtropical area in Southern China. Environ Res 186:109546
Mohammad KN, Chan EYY, Lau SY, Lam HCY, Goggins WB, Chong KC (2021) Relationship between acute kidney injury, seasonal influenza, and environmental factors: a 14-year retrospective analysis. Environ Int 153:106521
Mosili P, Maikoo S, Mabandla MV, Qulu L (2020) The Pathogenesis of Fever-Induced Febrile seizures and its current state. Neurosci Insights 15:2633105520956973
Nelson KB, Ellenberg JH (1976) Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med 295(19):1029–1033
Osilla EV, Marsidi JL, Sharma S (2022) In: StatPearls (ed) Physiology, temperature regulation. StatPearls, Treasure Island (FL)
Paul SP, Rogers E, Wilkinson R, Paul B (2015) Management of febrile convulsion in children. Emerg Nurse 23(2):18–25
Principi N, Esposito S (2013) Vaccines and febrile seizures. Expert Rev Vaccines 12(8):885–892
Sadleir LG, Scheffer IE (2007) Febrile seizures. BMJ 334(7588):307–311
Schneider A, Alexis NE, Diaz-Sanchez D, Neas LM, Harder S, Herbst MC, Cascio WE, Buse JB, Peters A, Devlin RB (2011) Ambient PM2.5 exposure up-regulates the expression of costimulatory receptors on circulating monocytes in diabetic individuals. Environ Health Perspect 119(6):778–783
Sharawat IK, Singh J, Dawman L, Singh A (2016) Evaluation of risk factors Associated with First Episode Febrile Seizure. J Clin Diagn Res 10(5):Sc10–13. https://doi.org/10.7860/jcdr/2016/18635.7853
Silvennoinen H, Huusko T, Vuorinen T, Heikkinen T (2015) Comparative burden of Influenza A/H1N1, A/H3N2 and B infections in children treated as outpatients. Pediatr Infect Dis J 34(10):1081–1085
Smith DK, Sadler KP, Benedum M (2019) Febrile seizures: risks, evaluation, and prognosis. Am Fam Physician 99(7):445–450
Verity CM, Butler NR, Golding J (1985) Febrile convulsions in a national cohort followed up from birth. I–Prevalence and recurrence in the first five years of life. Br Med J (Clin Res Ed) 290(6478):1307–1310
Vezzani A, Viviani B (2015) Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacol 96 (Pt A) 70–82. https://doi.org/10.1016/j.neuropharm.2014.10.027
Waruiru C, Appleton R (2004) Febrile seizures: an update. Arch Dis Child 89:751–756
Weichenthal S, Pinault LL, Burnett RT (2017) Impact of oxidant gases on the relationship between Outdoor Fine Particulate Air Pollution and Nonaccidental, Cardiovascular, and respiratory mortality. Sci Rep 7(1):16401
Xiong X, Wei Y, Lam HCY, Wong CKH, Lau SYF, Zhao S, Ran J, Li C, Jiang X, Yue Q, Cheng W, Wang H, Wang Y, Chong KC (2023) Association between cold weather, influenza infection, and asthma exacerbation in adults in Hong Kong. Sci Total Environ 857:159362
Zhao J, Gao Z, Tian Z, Xie Y, Xin F, Jiang R, Kan H, Song W (2013) The biological effects of individual-level PM(2.5) exposure on systemic immunity and inflammatory response in traffic policemen. Occup Environ Med 70(6):426–431
Acknowledgements
The authors thank the Hospital Authority, Hong Kong Observatory, and Hong Kong Environmental Protection Department for providing the data for analysis.
Funding
This work was supported by the National Natural Science Foundation of China (71974165) which will support open access publishing. This work is partially supported by the Health and Medical Research Fund (INF-CUHK-1, 19181132). The funder has no role in the study design, collection, analysis, and interpretation of data.
Author information
Authors and Affiliations
Contributions
Xiaoting Jiang: Conceptualization, Methodology, Formal analysis, Investigation, Writing - Original Draft. Conglu Li: Methodology, Writing - Review & Editing. Qianying Yue: Methodology, Writing - Review & Editing. Yuchen Wei: Methodology, Software, Writing - Review & Editing, Visualization. Yawen Wang: Methodology, Writing - Review & Editing. Xiang Qian Lao: Supervision, Writing - Review & Editing, Funding acquisition. Guozhang Lin: Methodology, Writing - Review & Editing. Ka Chun Chong: Conceptualization, Methodology, Writing - Original Draft, Supervision, Funding acquisition. All authors have read and approved the final paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study is approved by the Joint Chinese University of Hong Kong - New Territories East Cluster Clinical Research Ethics Committee. Informed consent was exempted as only aggregated data were used in this study.
Consent for publication
Not applicable.
Conflict of interest
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, X., Li, C., Yue, Q. et al. Effect of environmental exposome and influenza infection on febrile seizure in children over 22 years: a time series analysis. Int J Biometeorol (2024). https://doi.org/10.1007/s00484-024-02711-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00484-024-02711-8