Abstract
Key message
Tree species in a temperate floodplain forest had leaf turgor loss point values similar to those of upland forest trees, suggesting physiological drought tolerance in this generally non-water-limited system.
Abstract
Leaf turgor loss point (TLP) is a key plant trait associated with drought tolerance. In the bottomland hardwood (BLH) forests that grow in floodplains of the southeastern USA, drought stress is generally low but may increase with climate change. To address drought tolerance among BLH trees, we measured TLP among 20 species in a BLH forest in Louisiana, USA. We tested whether (1) TLP is higher in BLH tree species than in upland temperate-zone trees; (2) lower TLP is associated with higher drought tolerance among BLH species; (3) TLP drops during the growing season within BLH trees; and (4) within species, TLP is lower in more water limited, non-flooded BLH habitats than in seasonally flooded habitats. Among BLH tree species, TLP was −2.23 ± 0.28 (mean ± SD) and, contrary to our hypothesis, weakly positively correlated with drought tolerance. Within BLH species, TLP was lower in non-flooded habitats than seasonally flooded habitats and TLP decreased between the early and late growing season, more so in the non-flooded habitat. Overall, our results show that TLP among BLH trees is relatively low and plastic for a system that is generally not water limited, which may contribute to drought tolerance in future scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is a complex ecosystem disturbance that threatens forests globally (Brodribb et al. 2020). Numerous studies project increased drought severity associated with climate change (Trenberth et al. 2014). When droughts and heat waves reduce water availability, they often alter ecosystems. Extensive plant stress can lead to large forest mortality events, which are increasing in frequency (Allen et al. 2010; Choat et al. 2018). Widespread tree mortality can severely hinder the availability of forest resources to wildlife and humans, such as timber production and biodiversity (McDowell et al. 2020). Projecting these effects is a major challenge. Assessing variation in plant traits that are related drought responses can help to estimate ecosystem responses, for example by parameterizing dynamic vegetation models (Christoffersen et al. 2016; Powell et al. 2017).
In vascular plants, drought leads to higher xylem tension and an increased probability of embolism throughout the xylem network, causing vascular dysfunction (Brodribb and Cochard 2009; Choat et al. 2018). To survive droughts, trees must maintain their xylem tension below critical levels associated with catastrophic loss of stem hydraulic conductivity (Sperry and Love 2015). Generally, species-level traits determine species distributions when interacting with environmental variables, enabling spatial patterns in trait variation to explain patterns in community assembly (Bartlett et al. 2016). Improved quantification and understanding of plant traits across environmental gradients and spatial scales therefore have the potential to improve land models that predict forest distributions (Bartlett et al. 2014).
Plants depend on gradients in water potential for water uptake and growth (Meyer and Boyer 1972). Leaf turgor loss point (TLP) is the water potential at which leaf cells lose their turgor pressure and, generally, wilting occurs. Maintaining cell turgor above TLP is important for physiological function, including stomatal and hydraulic conductance, photosynthetic gas exchange, and growth under drought conditions (Brodribb and Holbrook 2003; Bartlett et al. 2014). At the leaf level, TLP is an indicator to varying degrees of drought tolerance within and across biomes (Bartlett et al. 2012b). Plants with lower TLP tend to have higher dehydration tolerance (sensu Volaire 2018), i.e., the ability to maintain physiological function at low plant water potential. In addition to low TLP, dehydration tolerance is associated with a suite of traits, such as the maintenance of stomatal- and hydraulic conductance low water potential (Bartlett et al. 2012a). In contrast, plants with high TLP vary in drought tolerance. While some are drought intolerant, other plants with high TLP are able to survive drought through dehydration avoidance (sensu Volaire 2018), i.e., the ability to maintain high plant water potential during drought conditions. Rather than TLP, dehydration avoidance is associated with other traits such as leaf shedding and dormancy (Bartlett et al. 2012a; Vargas et al. 2022). Thus, while no single trait is a perfect predictor of drought tolerance, TLP is informative, especially for assessing drought tolerance conferred through dehydration tolerance.
Plant species range widely in TLP and, within species, plants adjust their TLP in response to changing environmental conditions and seasons (Bartlett et al. 2014, 2016; Sjöman et al. 2018; Sueltenfuss et al. 2020). In water-limiting conditions, many plant species reduce TLP by increasing cytoplasmic solute concentrations within existing leaves or by developing new leaves with greater solute concentrations (Meyer and Boyer 1972; Bartlett et al. 2014; Sjöman et al. 2018). This enables leaves to remain turgid at low leaf water potentials that are required for maintaining a pressure gradient between drying soil and the canopy, which is required for the transpiration stream.
Floodplain forests are one of the most rapidly disappearing ecosystems in the world (Mikac et al. 2018). In the southeastern United States, floodplain forests are referred to as bottomland hardwoods (BLH). BLH forests occur extensively within the Mississippi Alluvial Valley, and are created and shaped through interactions with hydrological and alluvial geomorphological processes (Hodges 1997; Hupp 2000; King and Keim 2019). Most floodplains have topographic variation that results from stream meanders and differences in depositional patterns. Even slight elevational variations create hydrological regimes and stratification of tree species composition within BLH forests (Hodges 1997). To date, details regarding the effects of increased flooding and drought events on forest function within BLH forests are still largely unknown (King and Keim 2019). Commonly, artificial levees, dams, and other flood control actions alter or eliminate riverine flooding (Wharton et al. 1982; Gee 2012; Kroschel et al. 2016). Where present, levees disconnect floodplains from riverine flooding which has caused surface flooding to be largely dependent on precipitation (Gergel et al. 2002; Gee 2012; Kroschel et al. 2016). Similar to other temperate ecosystems, floodplain forests often get drier later in the growing season due to high air temperature and a decrease in the riverine input; however, high water tables and adequate rainfall make water limitation rare (Gee et al. 2014). This unique quality, together with their sensitivity to hydrological alterations, creates urgency in understanding the resistance of BLH species to increases in anthropogenic hydrological modifications and extreme climate events.
To date, very few BLH tree species have been measured for drought-response traits and drought tolerance. Here, we tested whether patterns in TLP that have been observed at the global and local scales in other forest types also occur in BLH forest. We measured TLP among tree species, seasons, and habitats in BLH forest in the Mississippi Alluvial Valley. Specifically, we tested the following hypotheses: (1) TLP is higher in BLH trees than in other temperate forest trees; (2) BLH tree species classified as highly tolerant to drought exhibit lower (i.e., more negative) values of TLP; (3) BLH trees have higher TLP in the early growing season than in the late growing season; and (4) within species, TLP is lower in the more water limited, non-flooded BLH habitats than in seasonally flooded habitats.
Materials and methods
Study site and sampling design
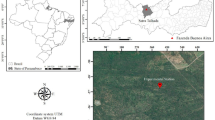
Leaf samples were collected at the Richard K. Yancey Wildlife Management Area (YWMA) located in Concordia Parish, Louisiana, USA (31° 4′ 9.7464″ N, 91° 40′ 21.2592″ W; Fig. 1). YWMA is a 28,681 ha protected area managed by the Louisiana Department of Wildlife and Fisheries (LDWF). The site is located between the Mississippi and Red Rivers beginning north of Lower Old River (Louisiana Department of Wildlife and Fisheries (LDWF) 2021). Mean precipitation is 140 cm y−1 (Gee et al. 2014). The site is a temperate deciduous forest with leaf flush in March–April to and leaf fall in October–November. The forest is dominated by American elm (Ulmus americana), green ash (Fraxinus pennsylvanica), sugarberry (Celtis laevigata), eastern cottonwood (Populus deltoides), American sycamore (Platanus occidentalis), and boxelder (Acer negundo) canopy species (LDWF 2021). Within the site, distinct plant communities are present corresponding with the various geomorphic features (e.g., swamps, sloughs, flats, and river fronts) that are commonly found in a major floodplain forest system (Hodges 1997). Two habitats were used for this study: (1) The non-flooded habitat (outlined in red in Fig. 1) is an area within a ring levee that prevents riverbank flooding, and (2) the seasonally flooded habitat (outlined in yellow in Fig. 1) is located outside the levees (i.e., batture lands) and is annually flooded by the Mississippi River during early growing season (generally from February to May) (Gee 2012). During the 2021 growing season (the study period), the seasonally flooded habitat was flooded until 24 May (E.A. Eisley, personal observation).
Map of study area located in Richard K. Yancey Wildlife Management Area (YWMA) in Concordia Parish, Louisiana, USA. The red and yellow boundaries delineate the non-flooded and seasonally flooded habitats within YWMA. Brown and blue circles represent trees that were sampled in the non-flooded and seasonally flooded habitats, respectively
Twenty BLH tree species that are common within YWMA were chosen for TLP measurement to survey a wide range of drought tolerances as described by Niinemets and Valladares (2006) (Table 1) and habitat preferences along hydrological and topographical regimes as described by Hodges (1997). Ten trees per species were measured, five each in the seasonally flooded and non-flooded habitat. Two species (Carya aquatica and Quercus lyrata) did not grow within the batture along the river, and therefore were excluded from sampling within the seasonally flooded habitat. Five species (C. aquatica, Gleditsia triacanthos, Salix nigra, Q. lyrata, and Taxodium distichum) were excluded from remeasurement in the late growing season due to time constraints. Each sample tree was measured once for diameter at breast height (DBH) in October 2021. DBH ranged from 1.5 to 100 cm, with an average DBH of 26 cm across species.
Fully expanded, sun exposed leaves were collected in the early growing season, soon after the seasonally flooded habitat was drained (1 June to 12 July 2021). Collection was repeated in the late growing season before leaves began to senesce (12 September to 31 October 2021). Leaves were collected by cutting branches down from target trees with hand pruners, a pole saw, or a 22-caliber rifle. After collection, branches were sealed in opaque plastic bags with wet paper towels and transported to the laboratory. Three leaves were sampled from each tree in each growing season, resulting in a total of 990 leaves sampled from 185 trees.
Leaf turgor loss point (TLP) measurement
Excised branches were recut underwater at least two nodes distal to the original cut. To saturate the leaves, the cut end of the branch was left submerged in a bucket with distilled water while the entire branch was sealed in the airspace above the water with opaque bags over night. To calculate TLP, osmotic potential at full turgor (πosm) was measured as described by Bartlett et al. (2012a). Leaf samples were screened to exclude any necrotic spots from sampling, patted dry with paper towels, and wiped clean of any soil or epiphylls. Two 6.8-mm-diameter discs were extracted from each leaf with a cork borer. The discs were tightly wrapped in foil and placed in liquid nitrogen for two-minutes to fracture cell membranes (Bartlett et al. 2012a). Samples were then sealed in plastic bags and stored in a −18 °C freezer, and in a −80 °C freezer for long term storage. One disc was measured for πosm with a vapor pressure osmometer (VAPRO 5520, Wescor, Logan, UT) following the protocol of Bartlett et al. (2012a). Immediately before inserting the sample into the osmometer chamber well, the foil was removed, and the leaf disc was punctured rapidly with a needle for an average of 10 s to achieve an even spread of punctures throughout the leaf disc. Each πosm measurement took an average of 10–15 min, with readings taken every 2 min until equilibrium was indicated by a decrease of <0.0125 MPa. The second “backup” disc was measured for TLP calculation if the first measurement failed (N = 14). TLP was then calculated by converting measured values of πosm to TLP using the equation developed by Bartlett et al. (2012a).
Statistical Analyses
All analyses were performed with R version 4.0.2 (R Core Team 2022). A total of 9 TLP samples were excluded from the dataset due to measurement error, sample misplacement, or when measured values of TLP were >3 SD from the species mean, leaving a sample set of a total of 981 TLP measurements. To test whether TLP was higher among BLH species than among upland temperate-zone species, we used a one-sided t-test to compare species-level means among our BLH samples and the TLP values of the 68 temperate-zone angiosperm and conifer species reported by Bartlett et al. (2012b). The Bartlett et al. (2012b) TLP dataset is a compilation of TLP values for 317 plant species from 72 studies. The TLP values compiled by Bartlett et al. (2012b) were measured with the pressure–volume curve technique whereas we measured TLP with the osmometer technique. However, the two techniques produce similar values (Bartlett et al. 2012a), enabling direct comparison.
To test whether TLP was associated with drought tolerance among BLH species, we used Pearson’s correlation analysis between species-level TLP values and their drought tolerance. We used the scores from the drought tolerance index of Niinemets and Valladares (2006). This index was constructed by combining and cross-validating data from various sources that ranked tolerances including expert opinions and comparative studies (Niinemets and Valladares 2006). The index gives species scores for drought tolerance on a continuous scale that ranges from 1 (very intolerant) to 5 (very tolerant). For comparison, we also tested the TLP-drought tolerance relationship among woody species from a wide range of temperate-zone biomes, including angiosperms and conifer forests, Mediterranean/temperate-dry biomes, and semi-deserts by combining the TLP values from Bartlett et al. (2012b) and the drought tolerance scores from Niinemets and Valladares (2006). We used Pearson’s correlation analysis on the 33 species for which both TLP and drought tolerance were reported (Table S1).
We evaluated how species, habitat, season, and DBH affected TLP with linear mixed-effects models using the lme4 package (Bates et al. 2015). Species, season, habitat, and their interactions were fixed effects. DBH, a proxy for tree size, was included as a fixed effect. Sample tree was a random effect because measurements were repeated within trees between seasons. The three leaf samples per tree and season were averaged, producing two measurements per tree that were included in the model (i.e., one from the early growing season and one from the late growing season). Because the distribution of TLP was approximately normally distributed, a Gaussian error distribution was used. Models were fit using maximum likelihood. Model selection was performed using the Akaike’s information criterion (AIC; Burnham and Anderson 2002), where the model with the lowest AIC value was designated as having the best fit. In cases where two or more models had AIC values within two units of one another, the model with the fewest parameters was retained. Additionally, marginal R2 (R2m), i.e., the variance explained by the fixed effects, and conditional R2 (R2c), i.e., the variance explained by the entire model, were used to assess model fit. R2m and R2c were calculated with the ‘MuMIn’ package (Bartoń 2022). We used the ‘ggeffects’ package (Lüdecke 2018) to extract the least square means of the coefficients in the final model. Finally, the relative contributions of species, habitat, tree, and season to variation in TLP were quantified with variance partitioning analysis using the nested random effects analysis described by Sokal and Rohlf (1995).
Results
TLP was highly variable within and among tree species (Fig. 2). Among all the leaves in the dataset, TLP ranged from −3.9 to −1.2 MPa (Fig. 2, Table S2) and species-level means ranged from −2.93 to −1.73 MPa (Table 1). We found no significant difference between species-level TLP in our BLH site and upland temperate-zone species reported by Bartlett et al. (2012b) (−2.23 ± 0.28 MPa vs. −2.19 ± 0.54, T = −0.44, P = 0.33). Among BLH species, there was a weak, positive correlation between drought tolerance and TLP (r = 0.46, P = 0.049; Fig. 3b). This trend held, with marginal statistical significance, when tested with TLP values from the early and late growing seasons in isolation (Fig. S1). In contrast, in the global dataset of upland temperate-zone species, TLP was negatively correlated with drought tolerance (r = −0.47, P = 0.006; Fig. 3a).
Boxplot of turgor loss point (TLP) for 20 tree species in a bottomland hardwood forest in Louisiana. Triangles and circles represent measurements on individual leaves taken during the early and late growing seasons, respectively. Blue and pink symbols represent samples collected in seasonally flooded and non-flooded habitats, respectively. Boxes enclose the 25th and 75th quartiles and are bisected by the medians. Bars extend to 1.5 times the interquartile range
The relationship between species-level turgor loss point (TLP) and drought tolerance among temperate upland species (a) and bottomland hardwood forest species (b). Circles represent species. TLP values in a and b are from Bartlett et al. (2012b) and the present study (Table 1), respectively. Drought tolerance scores are from Niinemets and Valladares (2006). Values of r and P are for Pearson’s correlation analyses. Trendlines are drawn to show the direction and strength of the relationships; they represent least squares linear regressions and 95% confidence intervals
The best fit linear mixed model, which tested for factors associated with variation in TLP, included species, season, habitat, DBH, and the interaction between season and habitat as fixed effects (Model 11 in Table 2, Table S2, Fig. 4). Including the interactions between species and habitat or species and season did not improve model fit (Table 2). Between the early and late growing seasons, the marginal mean TLP was predicted to drop from −2.18 MPa (95% CI = −2.22, −2.14 MPa) to −2.22 MPa (−2.27, −2.18 MPa) in the seasonally flooded habitat and −2.21 MPa (−2.25, −2.17 MPa) to −2.33 MPa (−2.38, −2.29 MPa) in the non-flooded habitat (Fig. 4a). TLP was predicted to decrease with DBH with a slope of −0.003 MPa cm−1 (−0.0042, −0.0017 MPa cm−1) (Fig. 4b). Variance partitioning analysis showed that 79.7% of the variance in TLP was explained by species; 0.07% by season within species; 0.55% by habitat within species and season; 15.2% by trees within species, season, and habitat; and 4.5% between leaves and measurement error.
Results from the best fit linear mixed-effects model (i.e., Model 11 in Table 2) of leaf turgor loss point (TLP) as a function of a growing season and habitat, and b diameter at breast height (DBH). Triangles and circles in a represent the least square means of the model coefficients in the early and late growing season, respectively, error bars represent 95% confidence intervals, and dashed lines connect species-level means (Table S1). In b, the black trend line represents the model coefficient for the relationship between TLP and DBH across species and the dashed lines represents the 95% confidence interval. The grey lines in b represent least-squares linear regressions fit to each species
Discussion
Our assessment of TLP among trees in a BLH provides insights into the physiological drought tolerance of a forest system that has rarely been exposed to drought conditions. Contrary to our expectation, we found that TLP was not higher in BLH species than global upland temperate-zone species, where water limitation is more prevalent (Gee et al. 2014). This suggests that BLH trees may be able to tolerate drought as well as upland trees. Indeed, when BLH sites become drier from levee construction, forest die off has rarely been reported. Instead, levee construction has resulted in increased prevalence of shade-tolerant and flood-sensitive species such as sugarberry (Celtis laevigata) and red maple (Acer rubrum) (Denslow and Battaglia 2002; Gee et al. 2014; Kroschel et al. 2016).
The pattern of low TLP being associated with high drought tolerance has been observed and validated in numerous studies (Bartlett et al. 2012a, b; Maréchaux et al. 2015; Powell et al. 2017; Zhu et al. 2018; Sjöman et al. 2018; Álvarez-Cansino et al. 2022). Our analysis of upland tree species showed this consistent pattern (Fig. 3a). However, among BLH tree species, there was a correlation in the opposite direction, where high TLP was associated with high drought tolerance (Fig. 3b). There are possibly two nonexclusive explanations for the positive TLP-drought tolerance relationship among BLH tree species. First, the index of drought tolerance available for our system was based on expert opinion rather than empirical measurements (Niinemets and Valladares 2006) and may not accurately reflect drought responses in BLH forest trees. Second, high drought tolerance in species with high TLP may be conferred through dehydration avoidance rather than dehydration tolerance (Sun et al. 2020; Álvarez-Cansino et al. 2022). High TLP enables leaves to quickly lose turgor and slow water loss as leaf water potential declines, and thereby maintain a high relative water content within the leaf (Read et al. 2006; Bartlett et al. 2012b; Álvarez-Cansino et al. 2022). Maintaining a high relative water content enables plants to avoid irreversible damage to cells and xylem conduits (Bartlett et al. 2012b). BLH species with relatively high TLP and drought tolerance may follow a dehydration-avoidance strategy, but this has not been assessed. We suggest that experimentally testing drought tolerance and its underlying mechanisms among BLH species would provide a better comparison of the TLP–drought-tolerance relationship.
TLP is well documented as a species-level trait, meaning there is higher variance among species than within species (Lenz et al. 2006; Bartlett et al. 2016; Maréchaux et al. 2016). For example, similar to our results, Maréchaux et al. (2016) found that 87% of the variance in TLP a tropical rainforest was among species (within a total of 50 samples among 9 species). However, Schmitt and Boisseaux (2023) found that only 53% of TLP variance in a tropical forest was attributable to species (within a total of 100 samples among 10 species). TLP reduction within species and individuals during water-deficit conditions is an adaptive response and an important component of plant-water relations (Bartlett et al. 2014). We found that, within BLH species, TLP dropped 0.08 MPa, on average, from the early to late growing season (Fig. 4a). In a global meta-analysis of TLP plasticity before and after water-deficit conditions, Bartlett et al. (2014) found an average drop of 0.44 MPa among 283 wild and crop species, >fivefold larger than that of the BLH species we measured. Osmotic adjustment likely helps plants to maintain physiological processes during dry periods (Bartlett et al. 2014; Maréchaux et al. 2017; Sjöman et al. 2018). Our BLH study site does not generally experience a dry season (Gee et al. 2014), so lower-than-global-average TLP adjustment would be expected. However, the TLP adjustment was higher in the non-flooded habitat than in the seasonally flooded habitat (marginal mean = 0.03 MPa vs. 0.12 MPa; Fig. 4a). This suggests that the non-flooded habitat was more water limited than the seasonally flooded habitat. Although we did not detect significant differences among species in seasonal TLP adjustment (i.e., species-by-season interaction in Table 2), species-level trends were highly variable (Fig. 1a, Table S2). To forecast and manage forest dynamics under future climate scenarios, it will be informative to identify species that have a high degree of TLP plasticity (Allen et al. 2010; Sjöman et al. 2018).
In landscapes with heterogeneous water availability and air temperature, species that specialize in drier microhabitats generally exhibit a more negative TLP than co-occurring specialists in wetter microhabitats (Bartlett et al. 2014). For example, in a study on TLP patterns across topographic moisture gradients in a tropical moist forest, Kunert et al. (2021) found that evergreen and brevi-deciduous species that specialized on dry plateau habitats had lower TLP, whereas deciduous species had no TLP-habitat association. These results are consistent with past studies demonstrating the tendency of tropical evergreen species to be more dehydration tolerant than tropical deciduous species (Calkin and Pearcy 1984; Bartlett et al. 2014). Unfortunately, we lack detailed information on variation in species distributions among microhabitats in our study site and cannot connect our measurements of TLP to elevational or microhabitat associations within our system. However, within our BLH species, we found a relatively weak average TLP adjustment of −0.06 MPa between the seasonally flooded to non-flooded habitat (Fig. 4a, Table S2). This is consistent with the global trend for drier habitats to contain plants with lower TLP (Bartlett et al. 2012b).
We found a weak relationship between TLP and DBH, with TLP decreasing at a rate of 0.003 MPa cm−1 (Fig. 4c). Similarly, Schmitt and Boisseaux (2023) found that TLP decreased with DBH within 10 tree species in a French Guianan forest. Also, Maréchaux et al. (2016) found that TLP decreased between saplings and canopy trees within five Amazonian species. Within trees, leaf water potential generally decreases with height, forming a pressure gradient that drives the water movement in the transpiration stream against gravity and hydraulic resistance (Venturas et al. 2017). Since osmotic potential is a component of water potential and the driver of TLP (Bartlett et al. 2012b), TLP would be expected to decline with tree height. Additionally, taller trees may benefit more from low TLP because they experience greater atmospheric evaporative demand (McDowell et al. 2008; Maréchaux et al. 2016).
Overall, we found that BLH tree species shifted TLP lower when water was more limited (Fig. 4), consistent with the role of lower TLP facilitating physiological function as water becomes limited (Bartlett et al. 2012b, 2014). We also found that TLP among BLH tree species was similar to that of upland tree species, where water is more limited (Fig. 3). Together, these results suggest that BLH forests may be resistant against drying, at least to the degree of current upland forests. Since, BLH tree species with the highest TLP were ranked the most drought tolerant (Fig. 3), dehydration avoidance strategies may confer further resistance against drought. However, more research on other drought-tolerance traits in BLH tree species, such as rooting depth and hydraulic vulnerability, is needed to inform these predictions.
Data availability
The data used in this study are available from the corresponding author upon reasonable request.
References
Allen CD, Macalady AK, Chenchouni H et al (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage 259:660–684. https://doi.org/10.1016/j.foreco.2009.09.001
Álvarez-Cansino L, Comita LS, Jones FA et al (2022) Turgor loss point predicts survival responses to experimental and natural drought in tropical tree seedlings. Ecology 103:e3700. https://doi.org/10.1002/ecy.3700
Bartlett MK, Scoffoni C, Ardy R et al (2012a) Rapid determination of comparative drought tolerance traits: using an osmometer to predict turgor loss point. Methods Ecol Evol 3:880–888. https://doi.org/10.1111/j.2041-210X.2012.00230.x
Bartlett MK, Scoffoni C, Sack L (2012b) The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: a global meta-analysis. Ecol Lett 15:393–405. https://doi.org/10.1111/j.1461-0248.2012.01751.x
Bartlett MK, Zhang Y, Kreidler N et al (2014) Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol Lett 17:1580–1590. https://doi.org/10.1111/ele.12374
Bartlett MK, Zhang Y, Yang J et al (2016) Drought tolerance as a driver of tropical forest assembly: resolving spatial signatures for multiple processes. Ecology 97:503–514. https://doi.org/10.1890/15-0468.1
Bartoń K (2022) MuMIn: multi-model inference. https://CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Brodribb TJ, Cochard H (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149:575–584. https://doi.org/10.1104/pp.108.129783
Brodribb TJ, Holbrook NM (2003) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol 132:2166–2173. https://doi.org/10.1104/pp.103.023879
Brodribb TJ, Powers J, Cochard H, Choat B (2020) Hanging by a thread? Forests and drought. Science 368:261–266
Burnham K, Anderson D (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer
Calkin HW, Pearcy RW (1984) Seasonal progressions of tissue and cell water relations parameters in evergreen and deciduous perennials. Plant, Cell Environ 7:347–352
Choat B, Brodribb TJ, Brodersen CR et al (2018) Triggers of tree mortality under drought. Nature 558:531–539. https://doi.org/10.1038/s41586-018-0240-x
Christoffersen BO, Gloor M, Fauset S et al (2016) Linking hydraulic traits to tropical forest function in a size-structured and trait-driven model (TFS vol 1-Hydro). Geosci Model Dev 9:4227–4255. https://doi.org/10.5194/gmd-9-4227-2016
Denslow JS, Battaglia LL (2002) Stand composition and structure across a changing hydrologic gradient: Jean Lafitte National Park, Louisiana, USA. Wetlands 22:738–752. https://doi.org/10.1672/0277-5212(2002)022[0738:SCASAA]2.0.CO;2
Gee H (2012) The effects of hydrologic modifications on floodplain forest tree recruitment and growth in the Mississippi River Alluvial Valley. LSU Doctoral Dissertations, Louisiana State University and Agricultural and Mechanical College
Gee HKW, King SL, Keim RF (2014) Tree growth and recruitment in a leveed floodplain forest in the Mississippi River Alluvial Valley, USA. For Ecol Manage 334:85–95. https://doi.org/10.1016/j.foreco.2014.08.024
Gergel SE, Dixon MD, Turner MG (2002) Consequences of human-altered floods: Levees, floods, and floodplain forests along the Wisconsin River. Ecol Appl 12:1755–1770. https://doi.org/10.1890/1051-0761(2002)012[1755:COHAFL]2.0.CO;2
Hodges JD (1997) Development and ecology of bottomland hardwood sites. For Ecol Manage 90:117–125. https://doi.org/10.1016/S0378-1127(96)03906-0
Hupp CR (2000) Hydrology, geomorphology and vegetation of Coastal Plain rivers in the south-eastern USA. Hydrol Process 14:2991–3010. https://doi.org/10.1002/1099-1085(200011/12)14:16/17%3c2991::AID-HYP131%3e3.0.CO;2-H
King SL, Keim RF (2019) Hydrologic modifications challenge bottomland hardwood forest management. J Forest 117:504–514. https://doi.org/10.1093/jofore/fvz025
Kroschel WA, King SL, Keim RF (2016) Tree regeneration by seed in bottomland hardwood forests: A review. Sena 15:42–60. https://doi.org/10.1656/058.015.sp907
Kunert N, Zailaa J, Herrmann V et al (2021) Leaf turgor loss point shapes local and regional distributions of evergreen but not deciduous tropical trees. New Phytol 230:485–496. https://doi.org/10.1111/nph.17187
Lenz TI, Wright IJ, Westoby M (2006) Interrelations among pressure–volume curve traits across species and water availability gradients. Physiol Plantarum 127:423–433.https://doi.org/10.1111/j.1399-3054.2006.00680.x
Louisiana Department of Wildlife and Fisheries (LDWF) (2021) Richard K. Yancey site description. https://www.wlf.louisiana.gov/page/richard-k-yancey. Accessed 15 Apr 2021
Lüdecke D (2018) ggeffects: tidy data frames of marginal effects from regression models. J Open Sour Softw 26:772. https://doi.org/10.21105/joss.00772
Maréchaux I, Bartlett MK, Sack L et al (2015) Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Funct Ecol 29:1268–1277. https://doi.org/10.1111/1365-2435.12452
Maréchaux I, Bartlett MK, Gaucher P, et al (2016) Causes of variation in leaf-level drought tolerance within an Amazonian forest. J Plant Hydraul 3:e004–e004. https://doi.org/10.20870/jph.2016.e004
Maréchaux I, Bartlett MK, Iribar A et al (2017) Stronger seasonal adjustment in leaf turgor loss point in lianas than trees in an Amazonian forest. Biol Let 13:20160819. https://doi.org/10.1098/rsbl.2016.0819
McDowell N, Pockman WT, Allen CD et al (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178:719–739. https://doi.org/10.1111/j.1469-8137.2008.02436.x
McDowell NG, Allen CD, Anderson-Teixeira K, et al (2020) Pervasive shifts in forest dynamics in a changing world. Science 368:eaaz9463. https://doi.org/10.1126/science.aaz9463
Meyer RF, Boyer JS (1972) Sensitivity of cell division and cell elongation to low water potentials in soybean hypocotyls. Planta 108:77–87. https://doi.org/10.1007/BF00386508
Mikac S, Žmegač A, Trlin D et al (2018) Drought-induced shift in tree response to climate in floodplain forests of Southeastern Europe. Sci Rep 8:16495. https://doi.org/10.1038/s41598-018-34875-w
Niinemets Ü, Valladares F (2006) Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol Monogr 76:521–547. https://doi.org/10.1890/0012-9615(2006)076[0521:TTSDAW]2.0.CO;2
Powell TL, Wheeler JK, de Oliveira AAR et al (2017) Differences in xylem and leaf hydraulic traits explain differences in drought tolerance among mature Amazon rainforest trees. Glob Change Biol 23:4280–4293. https://doi.org/10.1111/gcb.13731
R Core Team (2022) R: a language and environment for statistical computing
Read J, Sanson GD, de Garine-Wichatitsky M, Jaffré T (2006) Sclerophylly in two contrasting tropical environments: low nutrients vs. low rainfall. Am J Bot 93:1601–1614. https://doi.org/10.3732/ajb.93.11.1601
Schmitt S, Boisseaux M (2023) Higher local intra- than interspecific variability in water- and carbon-related leaf traits among Neotropical tree species. Ann Bot 131:801–811. https://doi.org/10.1093/aob/mcad042
Sjöman H, Hirons AD, Bassuk NL (2018) Improving confidence in tree species selection for challenging urban sites: a role for leaf turgor loss. Urban Ecosyst 21:1171–1188. https://doi.org/10.1007/s11252-018-0791-5
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. W.H. Freeman and Co., New York, NY
Sperry JS, Love DM (2015) What plant hydraulics can tell us about responses to climate-change droughts. New Phytol 207:14–27. https://doi.org/10.1111/nph.13354
Sueltenfuss JP, Ocheltree TW, Cooper DJ (2020) Evaluating the realized niche and plant–water relations of wetland species using experimental transplants. Plant Ecol 221:333–345. https://doi.org/10.1007/s11258-020-01015-2
Sun S, Jung E-Y, Gaviria J, Engelbrecht BMJ (2020) Drought survival is positively associated with high turgor loss points in temperate perennial grassland species. Funct Ecol 34:788–798. https://doi.org/10.1111/1365-2435.13522
Trenberth KE, Dai A, van der Schrier G et al (2014) Global warming and changes in drought. Nature Clim Change 4:17–22. https://doi.org/10.1038/nclimate2067
Vargas GG, Kunert N, Hammond WM et al (2022) Leaf habit affects the distribution of drought sensitivity but not water transport efficiency in the tropics. Ecol Lett. https://doi.org/10.1111/ele.14128
Venturas MD, Sperry JS, Hacke UG (2017) Plant xylem hydraulics: what we understand, current research, and future challenges. J Integr Plant Biol 59:356–389. https://doi.org/10.1111/jipb.12534
Volaire F (2018) A unified framework of plant adaptive strategies to drought: crossing scales and disciplines. Glob Change Biol 24:2929–2938. https://doi.org/10.1111/gcb.14062
Wharton CH, Kitchens WM, Pendleton EC, Sipe TW (1982) Ecology of bottomland hardwood swamps of the southeast: a community profile. Georgia Univ., Athens. Inst. of Ecology; Fish and Wildlife Service, Slidell, LA. National Coastal Ecosystems Team; Wabash Coll., Crawfordsville, IN. Dept. of Biology
Zhu S-D, Chen Y-J, Ye Q et al (2018) Leaf turgor loss point is correlated with drought tolerance and leaf carbon economics traits. Tree Physiol 38:658–663. https://doi.org/10.1093/treephys/tpy013
Acknowledgements
We thank Megan Bartlett for helpful advice on TLP measurement; Heidi Marshall, Zachary Mendheim, and Marisa Brennan for assistance in the field and laboratory; and two anonymous reviewers for helpful comments on the manuscript.
Funding
This project was supported by the National Institute of Food and Agriculture, US Department of Agriculture, under McInire Stennis project LAB94493.
Author information
Authors and Affiliations
Contributions
AE and BW designed the study. AE performed the data collection, performed data analyses, and wrote the first daft of manuscript. BW contributed to manuscript revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eisley, A.M., Wolfe, B.T. Leaf turgor loss point varies among tree species, habitats, and seasons in a bottomland hardwood forest. Trees 38, 263–272 (2024). https://doi.org/10.1007/s00468-023-02483-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-023-02483-5