Abstract
Background
Alport syndrome is a genetically heterogenous disorder resulting from variants in genes coding for alpha-3/4/5 chains of Collagen IV, which results in defective basement membranes in the kidney, cochlea and eye. The syndrome has different inheritance patterns and historically, was thought of as a disease affecting solely males.
Case
A 15-year-old female presented with pedal oedema, hypertension and proteinuria. She underwent a kidney biopsy which showed findings in keeping with focal segmental glomerulosclerosis. Her condition was refractory to steroids. Steroid-resistant nephrotic syndrome genetics were sent, revealing a rare pathogenic variant in the COL4A5 gene.
Conclusion
Heterozygous females with X-linked Alport syndrome can develop chronic kidney disease and hearing loss. Clinicians should be mindful when reviewing kidney histology to include Alport syndrome as a differential for female patients. COL4A3-5 genes should be included in all steroid-resistant nephrotic syndrome genetic panels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case
A 15-year-old female presented with a 2-week history of pedal oedema. She reported no antecedent illness, fever, arthropathy or rash. In early childhood, the patient had attended a general paediatrician for recurrent urinary tract infections. The patient had no significant family history. She had normal growth and development throughout her childhood. Her blood pressure was raised at 140/90 mmHg. Physical examination showed pedal oedema but was otherwise unremarkable. Urinalysis showed 3 + protein and microscopic haematuria. The following laboratory investigations were performed: Complete blood count; coagulation screen; blood urea nitrogen and creatinine; electrolytes; liver function tests; triglycerides, C3; C4; and ANCA. Her creatinine was 45 micromoles per litre, with an estimated glomerular filtration rate of 130 mL/min/1.73 m2. She was noted to be anaemic with a haemoglobin of 107 g/dL and a haematocrit of 0.31. Albumin was low at 23 g/L. Urinary protein: creatine ratio was elevated at 474 mg/mmol creatinine. The rest of the results were within normal limits.
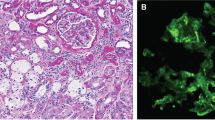
Her initial management included fluid restriction, diuretics and anti-hypertensive therapy. A subsequent kidney ultrasound was performed which was normal. She went on to have a kidney biopsy, prior to the commencement of steroids. She required steroids, diuretics and fluid restriction on discharge. The interval outpatient reviews showed persistence of pedal oedema, heavy proteinuria, hypoalbuminemia and hypertension. After two months of steroid therapy, a genetic panel for steroid-resistant nephrotic syndrome (SRNS) was sent. Her steroid therapy was stopped, and she started regular amlodipine, furosemide and tacrolimus. Her genetic test results revealed a definitive diagnosis. Her immunosuppression was stopped, she remained on diuretics and switched to an angiotensin receptor blocker. Her kidney biopsy histology can be seen in Fig. 1A–D.
Differential
This adolescent female patient experienced nephrotic-range proteinuria, hypoalbuminaemia, hypertension and haematuria. These findings are consistent with a mixed nephrotic-nephritic picture with a broad differential diagnosis. The differential includes, but is not limited to glomerulosclerosis, post-infectious glomerulonephritis, lupus nephritis and other causes of nephrotic/nephritic syndromes. Complement levels and vasculitis screening yielded no abnormal findings. Viral serology and anti-streptolysin titres were normal, making an infectious aetiology unlikely. Abdominal ultrasound, lactate dehydrogenase and urate were all normal, thus making a malignant cause unlikely. The patient had no recent medication or toxin exposure.
Biopsy showed marked effacement of the epithelial cell foot processes. There was some irregularity to the epithelial side of the basement membrane, but no other structural abnormalities were identified. These findings were in keeping with a diagnosis of focal segmental glomerulosclerosis, and as such, genetic studies for steroid-resistant nephrotic syndrome were performed. Immunofluorescence showed no evidence of an immune complex deposition disease process, thus excluding diagnoses such as IgA nephropathy, C3 glomerulopathy, lupus nephritis, etc. Electron microscopy revealed marked foot process effacement, consistent with a diffuse podocytopathy (Fig. 1A–D).
The selected steroid-resistant nephrotic syndrome genetic panel targeted 69 different genes using a custom designed SureSelect Target Enrichment System kit, with next-generation sequencing using a MiSeq (Illumina) analyser. Genetic studies on the patient revealed a heterozygous, likely pathogenic variant in the COL4A5 gene – c.3722G > A, p.(Gly1241Asp). At the time of presentation, this variant had not been reported in the literature. The c.3722G > A sequence change is predicted to cause the substitution of a highly conserved glycine residue for an aspartic acid residue at position 1241. Other amino acid changes at the same position have been reported in the literature in association with X-linked Alport syndrome: p.(Gly1241Arg), p(Gly1241Cys), and p.(Gly1241Val) [1, 2]. This was suggestive of a diagnosis of X-linked Alport syndrome. Parental genetics were performed, both of which were negative, suggesting that this variant arose de novo in this patient, supporting its pathogenicity.
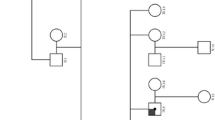
Following the diagnosis of X-linked Alport syndrome, the patient’s immunosuppression was stopped and she was commenced on an angiotensin receptor blocker. Given the normal parental genetics, the likelihood of our patient’s siblings being affected was low; however, the risk of gonadal mosaicism could not be fully excluded. As such, urinalysis and blood pressures were taken on all first-degree relatives, all of which were normal. She was transitioned to adult services with normal hearing and eye examinations.
Discussion
Alport syndrome is a genetically heterogenous disorder resulting from variants in genes coding for alpha-3/4/5 chains of Collagen IV which results in defective basement membranes in the kidney, cochlea and eye. The syndrome has different inheritance patterns: X-linked (COL4A5 mutation), autosomal recessive (homozygous or compound heterozygous mutations in COL4A3 or COL4A4 genes), and autosomal dominant (heterozygous mutations in COL4A3 or COL4A4 genes). Almost all males affected by X-linked Alport will progress to develop kidney failure, whereas just 12% of females with a heterozygous mutation progress to kidney failure [3].
This case was particularly unusual, given the age at presentation, the severity of her clinical presentation and the histology findings. Deng et al. described a case of an 11-year-old male, accompanied by a literature review, highlighting the importance of keeping Alport syndrome in the differential for both patients with glomerular haematuria and haematuria/proteinuria [4]. Historically, Alport syndrome has been thought of a disease affecting (almost exclusively) men. Alport himself stated that “the females have deafness and haematuria and live to an old age”. Perhaps the most detailed review of X-linked Alport syndrome in females was published by Michelle Rheault in 2011, outlining the progression of clinical knowledge through time, as well as collating the determinants of disease severity in heterozygous females with X-linked Alport syndrome (XLAS) [5]. Subsequent work by Quinlan and Rheault further details the incidence and likelihood of kidney failure in females with XLAS. Their review describes how ninety-five percent of women with XLAS have microscopic haematuria that may start at birth, with the median age of presentation with proteinuria being 7 years [6].
The main explanation for the variable severity of X-linked Alport syndrome in females is X-chromosome inactivation. Selective inactivation of the normal COL4A5 gene in kidney cells is thought to be a potential aetiology for severe Alport phenotype in heterozygous XLAS females. One previous paediatric case has been published outlining a female with aggressive early onset kidney failure secondary to this [7]. Ijjima and colleagues described a young female who presented initially at age two years, with nephrotic range proteinuria and haematuria. She went on to have a kidney biopsy at ages 6 and 8, with subsequent development of sensorineural hearing loss at the age of 14 years. Her diagnosis was made retrospectively at the age of 19 years, when she presented with microscopic haematuria, nephrotic-range proteinuria, hypoalbuminaemia and primary amenorrhoea [7].
Conclusion
Heterozygous females with X-linked Alport syndrome can develop chronic kidney disease and hearing loss. They should be followed by nephrologists in the same way their male counterparts are - surveillance with urinalysis, blood pressure monitoring, ophthalmology and audiology assessments are advisable. Further study into X-chromosome inactivation in tissue samples for Alport syndrome is warranted. Clinicians should be mindful when reviewing kidney histology to include Alport syndrome as a differential for female patients. COL4A3, COL4A4 and COL4A5 genes should be included in steroid-resistant nephrotic syndrome genetic panels.
Summary
What is new?
-
We present a rare case of X-linked Alport syndrome from a heterozygous, likely pathogenic variant in the COL4A5 gene–c.3722G > A, p.(Gly1241Asp).
References
Fallerini C, Dosa L, Tita R, Del Prete D, Feriozzi S, Gai G, Clementi M, La Manna A, Miglietti N, Mancini R, Mandrile G, Ghiggeri GM, Piaggio G, Brancati F, Diano L, Frate E, Pinciaroli AR, Giani M, Castorina P, Bresin E, Giachino D, De Marchi M, Mari F, Bruttini M, Renieri A, Ariani F (2014) Unbiased next generation sequencing analysis confirms the existence of autosomal dominant Alport syndrome in a relevant fraction of cases. Clin Genet 86:252–257. https://doi.org/10.1111/cge.12258
Boye E, Mollet G, Forestier L, Cohen-Solal L, Heidet L, Cochat P, Grunfeld JP, Palcoux JB, Gubler MC, Antignac C (1998) Determination of the genomic structure of the COL4A4 gene and of novel mutations causing autosomal recessive Alport syndrome. Am J Hum Genet 63:1329–1340
Jais JP, Knebelmann B, Giatras I, De Marchi M, Rizzoni G, Renieri A, Weber M, Gross O, Netzer KO, Flinter F, Pirson Y, Dahan K, Wieslander J, Persson U, Tryggvason K, Martin P, Hertz JM, Schroder C, Sanak M, Carvalho MF, Saus J, Antignac C, Smeets H, Gubler MC (2003) X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J Am Soc Nephrol 14:2603–2610
Deng Z, Zhou Q, Zhou TG (2023) A case report and literature study on Alport syndrome featuring nephrotic syndrome as its primary manifestation. Transpl Immunol 81:101941. https://doi.org/10.1016/j.trim.2023.101941
Rheault MN (2012) Women and Alport syndrome. Pediatr Nephrol 27:41–46. https://doi.org/10.1007/s00467-011-1836-7
Quinlan C, Rheault MN (2022) X-linked kidney disorders in women. Semin Nephrol 42:114–121. https://doi.org/10.1016/j.semnephrol.2022.04.002
Iijima K, Nozu K, Kamei K et al (2010) Severe Alport syndrome in a young woman caused by a t(X;1)(q22.3;p36.32) balanced translocation. Pediatr Nephrol 25:2165–2170. https://doi.org/10.1007/s00467-010-1514-1
Acknowledgements
The authors would like to thank the team at the Bristol genetics laboratory for their involvement and expertise in the diagnosis of this patient. The authors also thank the patient and her family for their permission to publish this case.
Funding
Open Access funding provided by the IReL Consortium
Author information
Authors and Affiliations
Contributions
DW, AF and AA conceptualised the manuscript, collected patient consent and data and were involved with the writing and revision of the manuscript. Authors CSC, RGS, AG and CS critically reviewed the manuscript for important intellectual content. All authors were involved in patient care.
Corresponding author
Ethics declarations
Patient consent
The patient has consented for the use of their data in this publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wildes, D.M., Fitzsimons, A., Doyle, B. et al. An unusual case of nephrotic syndrome. Pediatr Nephrol (2024). https://doi.org/10.1007/s00467-024-06408-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00467-024-06408-3