Abstract
Background
In the past decade, there have been substantial advances in our understanding of the pathobiology of pediatric acute kidney injury (AKI). In particular, animal models and studies focused on the relationship between kidney development, nephron number, and kidney health have identified a number of heterogeneous pathophysiologies underlying AKI. Despite this progress, gaps remain in our understanding of the pathobiology of pediatric AKI.
Methods
During the 26th Acute Disease Quality Initiative (ADQI) Consensus conference, a multidisciplinary group of experts discussed the evidence and used a modified Delphi process to achieve consensus on recommendations for opportunities to advance translational research in pediatric AKI. The current state of research understanding as well as gaps and opportunities for advancement in research was discussed, and recommendations were summarized.
Results
Consensus was reached that to improve translational pediatric AKI advancements, diverse teams spanning pre-clinical to epidemiological scientists must work in concert together and that results must be shared with the community we serve with patient involvement. Public and private research support and meaningful partnerships with adult research efforts are required. Particular focus is warranted to investigate the pediatric nuances of AKI, including the effect of development as a biological variable on AKI incidence, severity, and outcomes.
Conclusions
Although AKI is common and associated with significant morbidity, the biologic basis of the disease spectrum throughout varying nephron developmental stages remains poorly understood. An incomplete understanding of factors contributing to kidney health, the diverse pathobiologies underlying AKI in children, and the historically siloed approach to research limit advances in the field. The recommendations outlined herein identify gaps and outline a strategic approach to advance the field of pediatric AKI via multidisciplinary translational research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, significant advances spanning the research continuum have expanded our understanding of pediatric acute kidney injury (AKI). Basic science work explains kidney development and the diverse mechanisms of AKI, translational research extends these findings into clinical medicine, epidemiologic studies characterize the burden and clinical impact of the disease, and community interventions link these innovations to patients and their families.
Despite these advances, due to a number of challenges, there remain significant gaps in our understanding of the pathobiology of pediatric AKI. The diversity of “pediatric” patients ranges from premature neonates to adult-sized adolescents. A siloed approach to research and discovery in the field leads to a disconnect between bench, translational, and clinical research. Structural challenges exist in pediatric AKI research, including limited dedicated research funding and a lack of inclusion of pediatric or birth information in adult kidney studies.
The full ramifications of pediatric AKI remain unknown. The acknowledgment that AKI predisposes patients to developing chronic kidney disease (CKD) means that pediatric AKI survivors will become adults with CKD [1]. Beyond the kidney-specific outcomes of pediatric AKI across the life course, even less is known about the systemic sequelae that pediatric AKI may have on normal growth, development, and other organ dysfunction.
To address the need to improve research and clinical care in pediatric AKI, the 26th Acute Disease Quality Initiative (ADQI) conference was convened. We address three main questions from the conference in this article:
-
1.
What are the biopsychosocial factors that lead to optimal kidney development and to a healthy kidney lifespan?
-
2.
What are the biopsychosocial factors that lead to deviations from an optimal kidney life course?
-
3.
What are the necessary components to create an integrated framework for translational research to mitigate pediatric AKI and optimize lifelong kidney outcomes?
Methods
The 26th ADQI Consensus conference, the first ADQI devoted to pediatric AKI, was held over 3 days in Napa, CA, in November 2021, and included an interdisciplinary group of clinicians and researchers from North and South America, Africa, Asia, and Europe. Relevant disciplines were well represented, including pediatric nephrology, pediatric and adult critical care, pharmacy, epidemiology, health services research, advocates, pediatric nephrology nurses, nutritionists, and patients. As previously described, this consensus meeting followed the established ADQI process, with the broad objective to provide expert-consensus statements via interpretation of current knowledge for use by clinicians according to professional judgment and to identify evidence gaps to establish research priorities [2].
Workgroup 5 sought to develop consensus statements to improve future translational research in pediatric AKI and an understanding of the factors that lead to optimal kidney health and deviations from this optimal state. Given the large area of focus of workgroup 5 (pathobiology, pharmacology, and nutrition) and the task of answering 5 key questions, the 2 questions involving pharmacology and nutrition are addressed in a separate manuscript. The consensus-building process, informed by objective review of articles by workgroup members, used a modified Delphi method based on evidence when possible, with the ultimate goal of addressing the 3 key questions and articulating a research agenda to address existing knowledge gaps [3]. Consensus statements required two-thirds majority vote of all ADQI participants. Herein, we provide a summary of the current knowledge of factors impacting optimal kidney development and a healthy kidney lifespan, and more detailed recommendations regarding research approaches to be used as a framework for the advancement of pediatric AKI care.
Results
A critical recommendation of the consensus panel was a need to improve pediatric AKI research across the continuum from bench to bedside, as follows:
Successful pediatric translational AKI research programs include diverse teams using reverse translational approaches in partnership with clinical and epidemiological findings that prioritize development as a biologic variable. Sufficient support including pediatric specific government and industry funding along with meaningful partnerships among health professionals is necessary to understand and leverage the unique aspects of pediatric AKI to address kidney health and disease across the life course.
In order to fully address the current state of evidence leading this consensus recommendation and opportunities for future translational research advancement, we sought to answer the 3 questions related to pathobiology developed during the pADQI conference.
Question 1. What are the biopsychosocial factors that lead to optimal kidney development and to a healthy kidney lifespan?
Pediatric kidney disease research, and specifically pediatric AKI, is uniquely challenged due to the wide spectrum of progressive developmental states included within pediatric medicine. For example, a premature neonate and a post-pubertal teenager are both considered pediatric patients despite differences in development, nephron number, risk for, and impact of episodes of AKI on future health. In order to better understand the spectrum of pediatric AKI and to advance our understanding of the diverse pathobiology of this condition, we need to better understand development as a biologic variable (DABV) and the factors which contribute to maximum nephron number, kidney development, and kidney health throughout the lifespan. Nephrogenesis is complete at approximately 34 weeks gestation and kidney function continues to develop and mature throughout the first 2 years of life. Glomerular and tubular function undergo constant change and maturation throughout this period. Herein, we use DABV to assess the potential effect this flux in kidney development has on AKI incidence, severity, and outcomes (Table 1).
Current understanding of development as a biological variable
Kidney development and nephron number is a result of a complex interplay between distinct embryologically derived cell populations [4]. Many factors that appear to impact DABV, including the genetic and molecular regulation of kidney development and the mechanisms of kidney development, have been extrapolated from animal work [5, 6]. Additionally, the role of epigenetic programming in premature birth remains unclear. Recent animal studies have shown that perinatal epigenetic programming through alterations in the kidney corticosteroid signaling pathways may contribute to DABV following premature birth [7]. In addition, there is now compelling pre-clinical evidence for the induction of an embryonic phenotype in injured tubule cells of the adult kidney, with robust re-expression of genes normally present only in the developing kidney [8]. This switch to the embryonic state is likely critical for regeneration of tubule cells lost during AKI. The identified developmental genes and gene products that accelerate repair in the adult kidney represent novel future therapeutic targets.
Factors impacting nephron number
Factors impacting nephron number and development impact kidney health along the entire life course, and are therefore an issue of major consequence for pediatric AKI and long-term outcomes. There is wide variability in human nephron number as early as the neonatal period due to DABV [9]. In humans, the completion of nephrogenesis coincides with the completion of gestation; nephron number is therefore impacted by gestational age at birth [9]. The cessation of human nephrogenesis is relatively consistent across studies and has been reported to occur between 32 and 36 weeks’ gestation. However, kidney function continues to develop and mature throughout the first 2 years of life. Nephrogenesis was recently documented at 37 weeks’ gestation, and several adult studies have found a correlation between birth weight, glomerular number, and risk of CKD later in life [10, 11]. It is not currently possible to determine when nephrogenesis is complete in humans because the data regarding the window of nephrogenesis is derived exclusively from post-mortem studies [11]. The duration of human nephrogenesis is likely variable and may be a factor in an individual’s nephron endowment.
From an evolutionary perspective, fetal response to intrauterine stress is to reduce somatic growth and the growth of any organ not vital to early survival [12]. For the kidney, a “surplus” of nephrons is not likely to provide an early survival benefit. However, the trade-off for a lower initial nephron number is the increased risk for CKD as a person ages [13]. Beyond the many genes that confer a low nephron number and result in congenital anomalies of the kidney and urinary tract, low birth weight has been recognized as a risk factor for the development of CKD [14]. However, the specific mechanisms that influence nephrogenesis and final nephron number, both in utero and ex utero, and the processes regulating nephron loss are not well understood.
Any factor that mitigates optimal baseline kidney health likely predisposes patients to developing AKI and may also impair recovery. Hence, these factors, when perturbed early in life, may have a significant impact on kidney health across the lifespan.
Question 2. What are the biopsychosocial factors that lead to deviations from an optimal kidney life course path?
Biopyschosocial factors impacting nephron development
Many other factors influence nephron number in utero and ex utero [9]. Maternal protein restriction and deficiency of iron or vitamin A can reduce nephron endowment [15,16,17]. In animal models, periods of maternal fasting are associated with a reduction in nephron number in offspring [18], and this appears to be mediated in part by an associated congenital nephron deficit occurring from intrauterine growth restriction [19]. Reduced glomerular filtration rate and albuminuria accompany nephron reduction with numerous studies demonstrating an increased prevalence of microalbuminuria and proteinuria among adults born low birth weight [20,21,22]. It is challenging to separate birth weight and gestational age from low birth weight (LBW), which is often used as a surrogate marker of prematurity [23]. In epidemiologic studies, food insecurity, a social determinant of health associated with malnutrition, was associated with higher rates of CKD and faster progression to kidney failure [24, 25]. More studies are needed to assess the role of maternal malnutrition and food insecurity in nephron number and recovery from AKI in children.
Over time, nephron number appears to naturally decrease in humans. In the last decade, investigators have capitalized on the unique setting of living donor kidney transplantation to study nephron number, finding an estimated average glomerular number in healthy donors of nearly 900,000, and have shown a lower glomerular number in kidney donors of older ages [26]. These data are limited by the mostly cross-sectional nature of epidemiologic studies. The potential impact of nephron development during infancy and early childhood on age-related kidney function decline remains unknown.
Biopsychosocial contributions to nephron number
In the 1980s, Brenner proposed that low nephron endowment would lead to impaired sodium excretion, glomerular hypertrophy, and glomerular hypertension, leading to the subsequent development of glomerulosclerosis and further decline of nephron number [27]. This hypothesis has not been tested in vivo. In preclinical model of LBW, those born LBW had a greater proportional increase in kidney size and glomerular hypertrophy compared to normal birth weight controls [28]. In settings of reduced nephron number, this compensation may be exaggerated and could lead to accelerated loss of kidney function [29, 30]. There is still a great deal to understand regarding the thresholds of nephron number to cause hypertrophy and the capacity and limit of glomerular hypertrophy.
Greater severity or more rapid progression of kidney disease has been shown among adults born with LBW and/or prematurity [27]. The incidence of adult kidney failure is 40% greater among those with birth weights under 2.5 kg compared to those with normal birth weight [31]. Children born LBW had lower glomerular density with glomerular enlargement on kidney biopsy compared to those born at normal birth weight [32]. Focal segmental glomerulosclerosis (FSGS) is the predominant histologic finding in kidney biopsies of adolescents and adults born preterm [33]. In children with FSGS on biopsy, those with LBW had hyperplastic glomeruli with fewer podocytes and more sclerotic lesions [16, 34]. Taken together, these findings suggest the concept of a “podocytopathy” from preterm birth [11, 12, 33, 35] or growth restriction, which may contribute to the variety of outcomes seen in patients with nephrotic syndrome, FSGS, or IgA. Biopsychosocial variables impact the risk of preterm birth, and these same variables may continue to impact kidney development after birth.
AKI in development of kidney dysfunction
The risk of CKD or kidney failure after AKI has been well detailed in adult studies [36]. In 2019, an updated meta-analysis and systematic review identified 82 studies including over 2 million adult patients who had AKI [37]. Following an episode of AKI, adults had a hazard ratio (HR) of 2.67 for new or progressive CKD (CI 1.99–3.58), 4.81 for kidney failure, and 1.8 for death.
Researchers have proposed that AKI and CKD are interconnected syndromes, and not separate disease processes [38]. The mechanisms for progression to CKD are incompletely understood, but likely are secondary to maladaptive repair, ongoing inflammation, and disordered regeneration [39]. The evidence of progression to CKD from AKI is less established in pediatric patients. In a systematic review of 346 patients with a mean follow-up of 6.5 years, the cumulative incidence of abnormal GFR < 90 mL/min/1.73 m2 was 6% [40]. Similarly, 10% of pediatric patients had CKD (defined as albuminuria and/or GFR < 60) 1 to 3 years following AKI, while 47% were at risk for CKD (defined as GFR < 90, hypertension or GFR > 150) [41, 42]. In 100 pediatric patients with nephrotoxin-associated AKI, 70% had evidence of residual kidney damage 6 months after AKI [43]. These studies suggest that the impact an episode of early childhood AKI has on long-term kidney health is likely different than the impact of AKI on adults with fully developed kidney function. These impacts likely change significantly with respect to kidney DABV. Larger pediatric studies that include consistent AKI and CKD definitions are needed in order to define the absolute risk of CKD development after childhood AKI.
A thorough review of translational AKI models has recently been published [44]. These models provide a robust platform to investigate the various pathophysiologies of AKI (i.e., sepsis, ischemia–reperfusion, nephrotoxic). However, most pre-clinical models do not account for the additional complexity of pediatric AKI imparted by DABV. Furthermore, the effect of an episode of AKI during various stages of kidney development remains unclear. We propose that the severity, duration, and timing of an AKI episode with respect to DABV impact the long-term kidney and global health outcomes across the child’s lifespan. DABV can be investigated by using models that incorporate kidney development. For example, Chevalier et al. developed a partial-reversible unilateral ureteral obstruction model in rat pups that enables the investigation of clinically relevant partial obstruction in neonates [45]. Liberio et al. recently published new models of pediatric ischemia–reperfusion and nephrotoxic AKI in rat pups and demonstrated the kidney-lung crosstalk in pulmonary vascularization [46]. Stem cells and organoids can be investigated using reverse-translational approaches spanning the pathway of nephron and organoid development [47]. Such models can be used to probe the unique aspects of pediatric AKI compared to AKI in adults.
Sex as a biologic variable
Sex is an important biologic variable in the development of AKI, and progression to CKD [48,49,50,51,52]. The NIH has made a call to action for the inclusion of both sexes in pre-clinical and clinical research [53]. Established sex differences in AKI (e.g., female sex is protective in ischemia–reperfusion AKI and deleterious in some forms of nephrotoxin-mediated AKI) have stymied the inclusion of both males and females in pre-clinical animal studies. Determining the mechanism of protective sex biases would allow researchers to identify novel therapeutic targets that benefit both sexes.
In pediatrics, sex as a biological variable (SABV) is further confounded by DABV. Hormone levels change significantly from pre-pubertal to peri-pubertal to post-pubertal stages, and few clinical studies capture pubertal stage or measure sex hormones. Complexity is further added for the care of intersex and transgendered youths undergoing sex affirming hormonal therapy. Pre-clinical animal models exist which unconfound the effects of sex hormones (estrogen and testosterone) from sex chromosomes (XX, XY) [44]. Using models which take into account SABV incorporating DABV could be applied to inform translational studies in a diverse population of patients along the gender spectrum.
Investigating systemic and long-term outcomes after AKI
Most pre-clinical AKI research has been confined to relatively short-term outcomes. Additionally, there is a growing appreciation that AKI results in systemic sequelae [54]. Studies have shown that premature infants with AKI have worse short- and long-term pulmonary outcomes and neurologic outcomes [55, 56]. Recent preclinical studies demonstrate long-term cardiovascular and growth effects after ischemia–reperfusion AKI [37, 57, 58]. Septic AKI is associated with worse functional outcomes; this same observation has been demonstrated in survivors of continuous kidney replacement therapy [59,60,61]. The potential effects of AKI with respect to DABV on these long-term outcomes are unknown and may have significant implications for global health outcomes of children who suffer an episode of AKI. Preclinical models specific to pediatrics are needed to bridge these knowledge gaps.
Question 3: What are the necessary components to create an integrated framework for translational research to mitigate pediatric AKI and optimize lifelong outcomes?
An integrated approach to translational research
AKI is a syndrome with heterogeneous causes and multiple clinical phenotypes, which require a detailed understanding of DABV. Traditionally, translational AKI research has used numerous pre-clinical models to investigate the mechanisms of AKI pathophysiology and outcomes with a goal to bring potential therapies to the bedside [44]. Until recently, modeling of AKI was primarily based on animal and cell culture models. Numerous animal models of AKI have been established, each with translational strengths, advantages, and challenges. However, these models of AKI differ significantly from human AKI in molecular and cellular responses, biomarkers, and clinical manifestations and transferring animal and pre-clinical data from animal models to humans is inherently challenging [62, 63]. In addition to the limitations of translating animal models to human research, translating these animal models for pediatric AKI faces additional challenges: (1) the vast majority of preclinical AKI models evaluate short-term outcomes, and (2) these models are performed in young adult animals. As a result, the standard preclinical AKI model is unable to recapitulate the unique aspects of growth and development in pediatric medicine and also fails to capture the important aspect of the long-term systemic and kidney sequalae of AKI along the life course.
The past two decades have seen a paradigm shift, moving towards personalized human-based models to study human disease. Novel in vitro systems for AKI diagnosis include human-induced pluripotent kidney stem cells [64,65,66], human stem cell-derived kidney organoids [67, 68], and human kidney tumor-derived stem cells [69]. Preclinical models of AKI have resulted in significant advances in the pathophysiology of AKI and identified several non-dialytic therapeutic targets which include the following [70, 71]: anti-inflammatory [72, 73], anti-necrosis/apoptosis [74, 75], antioxidants [76], anti-sepsis [77], growth factors [78, 79], and vasodilators [80, 81]. Additionally, prevention of AKI has been demonstrated with methylxanthines [82, 83]; in particular, caffeine administration may reduce the risk of AKI in premature neonates [84]. More novel therapeutics such as mesenchymal stem cells (MSCs) have also been shown to improve outcomes in AKI [85, 86]. Numerous injectable hydrogel systems have been studied for local delivery of therapeutics to the kidney [87,88,89,90,91,92,93]. These injectable systems allow for sustained local delivery over a set period of time [94]. Several potential therapeutics are currently undergoing clinical trials [95].
Despite these advances, there have been challenges translating therapies into clinical use, and many promising preclinical therapies have failed to demonstrate efficacy in human trials [96]. The NIDDK published guidelines to overcome these barriers, and recommends utilizing a reverse-translational approach whenever possible [97]. In this manner, the preclinical models are designed to match the clinical intervention with respect to disease pathophysiology, timing of intervention, and outcome measures. The group also recommended incorporation of serum and urine biomarkers in addition to functional assessments of kidney function to better translate the spectrum of kidney injury between preclinical and clinical studies [97].
To improve the translational impact of these research efforts, we advocate for an approach that integrates pre-clinical, clinical, and epidemiological research efforts so that they can inform one another in study design and outcome measures and that pediatric-specific pre-clinical models are employed whenever available (Table 2).
Diversity increases innovation
Diverse teams improve innovation in research and clinical delivery of pediatric AKI care. Beyond the diversity of expertise required to inform and perform translational AKI research, teams are further strengthened by the diversity of their background, including but not limited to, sex, gender, age, race, ethnicity, and ability [98]. Empirical data demonstrate that diverse research teams produce more innovative research; yet, publications from authors who identify as under-represented in medicine are less likely to be highly cited [98, 99].
Research investigations are strengthened by the inclusion of team members with a broad array of expertise, such as the inclusion of clinical researchers on pre-clinical work and health service researchers, patients and patient advocates, and implementation scientists to understand how to optimally deploy clinical and research findings [44]. A truly integrated research approach includes not only investigations throughout the basic science to epidemiologic spectrum, but also incorporates digital health tools and community partnerships to better meet patients and families in their communities and make them partners in health [100]. Health service and epidemiological studies may benefit from inclusion of specific outcome measures guided by pre-clinical work. Conversely, epidemiological findings can drive pre-clinical mechanistic investigations via reverse-translational approaches [63]. In addition, partnerships between patients, patient advocates, clinicians, researchers, industry, and policy makers can help identify core outcomes. Such outcomes developed on shared priorities have the potential to make research more meaningful for the patients, and the clinicians taking care of them [101].
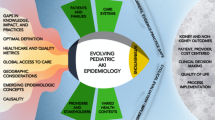
Beyond the research setting, research teams must successfully disseminate their findings and engage key stakeholders in order to improve care and serve the community. This includes sharing new knowledge in context-appropriate ways with members of the healthcare team, patients, families, and the community [3]. This need is discussed in further depth by Workgroup 6. Collaborations among research teams, educators and community representatives may enable innovative approaches to reach the communities they benefit (Fig. 1). Engagement of government and funding agencies in such efforts can increase awareness, and potentially improve resources available to healthcare teams [102].
Visual representation of an integrated framework for translational research, including the multiple silos of research including preclinical research, reverse translation approaches, and clinical research. Along with community engagement and digital health, these elements all interact in a multi-directional collaborative approach to impact pediatric AKI care
To bolster diversity and innovation in pediatric AKI research, individual, institutional, and system-wide support is needed to recruit, train, support, retain, and amplify a robust pipeline of translational researchers from diverse backgrounds [103]. Diversity in the makeup of research and clinical teams should be deliberately supported, encouraged, and rewarded by research enterprises and institutions, with specific attention paid to efforts of equity and inclusion. The Society for Pediatric Research recently published a Call to Action with specific and meaningful ways academic organizations, schools, departments, and faculty members can improve the diversity of the pediatric scientific workforce [104].
Adequate funding and investment
In order to advance our understanding of pediatric AKI and identify therapeutic targets to optimize outcomes throughout the life course, robust, sustained, and predictable funding support is required. As a whole, kidney disease research is underfunded, with an NIH investment of < 1% of the cost of kidney care [105]. This inequity in funding is likely even more stark in pediatric kidney disease research. However, it is not clear how much support pediatric kidney disease research currently receives, because this has not been tracked by the NIH.
The NIDDK has recently announced its 5-year strategic plan to augment kidney disease research, which does not specifically prioritize or target pediatric kidney disease research. Beyond the USA, more global investment is warranted. Currently, the global action plan for the prevention and control of noncommunicable diseases does not include kidney disease; we join others in advocating for its inclusion [106]. In addition to research funding from governments and institutions, public–private partnerships and industry involvement should be encouraged. Appropriately designed regulations and incentives can foster investment into pediatric research by biopharmaceutical companies.
Pediatric kidney research should be prioritized given its impact on patients throughout their life course. Additionally, as developmental pathways are reactivated during and after AKI, a better understanding of these pathways is essential for better understanding of recovery from AKI versus progression to CKD. In light of the burden of AKI and CKD in the adult population, investments in understanding these developmental pathways may be an efficient use of limited research funding. Kidney-related outcomes should be a focus of not only NIDDK studies but should be included in other pediatric studies and clinical trials, including targeted support from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [107]. During the early neonatal period and childhood is the only time where nephron number can be impacted, either optimizing this for the life course or setting up a child for a lifetime risk of decreased nephron number and increased risk of CKD. Additionally, as pediatric patients with AKI are at-risk to become adults with CKD, and as seriously ill children are future adult patients, aligning and combining research efforts in adult and pediatric nephrology would benefit both fields and improve outcomes for patients along the life course with kidney disease. Inclusion of children in clinical investigations and collaboration among researchers in various specialties optimizes the translatability of research investigations. For example, coordinated projects such as the Kidney Precision Medicine Project represent opportunities to include pediatric samples, and in doing so, broaden and bolster the research findings.
Conclusion
Despite recent advances in our understanding of the pathobiology of pediatric AKI, there remain large gaps in our understanding of the diverse spectrum of pediatric AKI. Further research advancements in the field require that pediatric translational AKI research programs focus on the unique aspects of development as a biological variable, and the impact of kidney health and disease across the life course. These efforts merit and require substantial support. In order to accomplish these goals, research must include diverse and multidisciplinary teams, be supported by robust and predictable funding from government and industry and employ meaningful partnerships with multiple other medical disciplines.
Data availability
This is not applicable to this manuscript as no new data was created.
References
Sato Y, Takahashi M, Yanagita M (2020) Pathophysiology of AKI to CKD progression. Semin Nephrol 40:206–215
Kellum JA, Palevsky P, Mehta R, Bellomo R, Ronco C (2002) Acute dialysis quality initiative: methodology. Curr Opin Crit Care 8:500–501
Goldstein SL, Akcan-Arikan A, Alobaidi R, Askenazi DJ, Bagshaw SM, Barhight M, Barreto E, Bayrakci B, Bignall ONR, Bjornstad E, Brophy PD, Chanchlani R, Charlton JR, Conroy AL, Deep A, Devarajan P, Dolan K, Fuhrman DY, Gist KM, Gorga SM, Greenberg JH, Hasson D, Ulrich EH, Iyengar A, Jetton JG, Krawczeski C, Meigs L, Menon S, Morgan J, Morgan CJ, Mottes T, Neumayr TM, Ricci Z, Selewski D, Soranno DE, Starr M, Stanski NL, Sutherland SM, Symons J, Tavares MS, Vega MW, Zappitelli M, Ronco C, Mehta RL, Kellum J, Ostermann M, Basu RK; Pediatric ADQI Collaborative (2022) Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi consensus statement. JAMA Netw Open 5:e2229442
Chambers JM, Wingert RA (2020) Advances in understanding vertebrate nephrogenesis. Tissue Barriers 8:1832844
Volovelsky O, Nguyen T, Jarmas AE, Combes AN, Wilson SB, Little MH, Witte DP, Brunskill EW, Kopan R (2018) Hamartin regulates cessation of mouse nephrogenesis independently of Mtor. Proc Natl Acad Sci U S A 115:5998–6003
Li H, Kurtzeborn K, Kupari J, Gui Y, Siefker E, Lu B, Matlik K, Olfat S, Montano-Rodriguez AR, Huh SH, Costantini F, Andressoo JO, Kuure S (2021) Postnatal prolongation of mammalian nephrogenesis by excess fetal GDNF. Development 148:dev197475
Dumeige L, Nehlich M, Viengchareun S, Perrot J, Pussard E, Lombes M, Martinerie L (2020) Preterm birth is associated with epigenetic programming of transgenerational hypertension in mice. Exper Mol Med 52:152–165
Rudman-Melnick V, Adam M, Potter A, Chokshi SM, Ma Q, Drake KA, Schuh MP, Kofron JM, Devarajan P, Potter SS (2020) Single-cell profiling of AKI in a murine model reveals novel transcriptional signatures, profibrotic phenotype, and epithelial-to-stromal crosstalk. J Am Soc Nephrol 31:2793–2814
Charlton JR, Baldelomar EJ, Hyatt DM, Bennett KM (2020) Nephron number and its determinants: a 2020 update. Pediatr Nephrol 36:797–807
Ryan D, Sutherland MR, Flores TJ, Kent AL, Dahlstrom JE, Puelles VG, Bertram JF, McMahon AP, Little MH, Moore L, Black MJ (2018) Development of the human fetal kidney from mid to late gestation in male and female infants. EBioMedicine 27:275–283
Rodriguez MM, Gomez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE (2004) Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol 7:17–25
Chevalier RL (2017) Evolutionary nephrology. Kidney Int Rep 2:302–317
Luyckx VA, Brenner BM (2020) Clinical consequences of developmental programming of low nephron number. Anat Rec (Hoboken) 303:2613–2631
Eriksson JG, Salonen MK, Kajantie E, Osmond C (2018) Prenatal growth and CKD in older adults: longitudinal findings from the Helsinki Birth Cohort Study, 1924–1944. Am J Kidney Dis 71:20–26
Welham SJ, Wade A, Woolf AS (2002) Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int 61:1231–1242
Lisle SJ, Lewis RM, Petry CJ, Ozanne SE, Hales CN, Forhead AJ (2003) Effect of maternal iron restriction during pregnancy on renal morphology in the adult rat offspring. Br J Nutr 90:33–39
Lelièvre-Pégorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, Merlet-Bénichou C (1998) Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int 54:1455–1462
Mohany M, Ashton N, Harrath AH, Nyengaard JR, Alomar SY, Alwasel S (2018) A new model for fetal programming: maternal Ramadan-type fasting programs nephrogenesis. J Dev Orig Health Dis 9:287–298
Celsi G, Kistner A, Aizman R, Eklöf AC, Ceccatelli S, de Santiago A, Jacobson SH (1998) Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res 44:317–322
Vehaskari VM, Aviles DH, Manning J (2001) Prenatal programming of adult hypertension in the rat. Kidney Int 59:238–245
Yudkin JS, Martyn CN, Phillips DI, Gale CR (2001) Associations of micro-albuminuria with intra-uterine growth retardation. Nephron 89:309–314
Hoy WE, Rees M, Kile E, Mathews JD, McCredie DA, Pugsley DJ, Wang Z (1998) Low birthweight and renal disease in Australian aborigines. Lancet 352:1826–1827
Crump C, Sundquist J, Winkleby MA, Sundquist K (2019) Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 365:l1346
Banerjee T, Crews DC, Wesson DE, Dharmarajan S, Saran R, Ríos Burrows N, Saydah S, Powe NR (2017) Food insecurity, CKD, and subsequent ESRD in US adults. Am J Kidney Dis 70:38–47
Crews DC, Kuczmarski MF, Grubbs V, Hedgeman E, Shahinian VB, Evans MK, Zonderman AB, Burrows NR, Williams DE, Saran R, Powe NR (2014) Effect of food insecurity on chronic kidney disease in lower-income Americans. Am J Nephrol 39:27–35
Denic A, Lieske JC, Chakkera HA, Poggio ED, Alexander MP, Singh P, Kremers WK, Lerman LO, Rule AD (2017) The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol 28:313–320
Garrett PJ, Bass PS, Sandeman DD (1994) Barker, Brenner, and babies–early environment and renal disease in adulthood. J Pathol 173:299–300
Baldelomar EJ, Charlton JR, Beeman SC, Bennett KM (2018) Measuring rat kidney glomerular number and size in vivo with MRI. Am J Physiol Renal Physiol 314:F399–F406
Carmody JB, Charlton JR (2013) Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics 131:1168–1179
Chevalier RL (2020) Bioenergetic evolution explains prevalence of low nephron number at birth: risk factor for CKD. Kidney360 1:863–879
Lackland DT, Egan BM, Syddall HE, Barker DJ (2002) Associations between birth weight and antihypertensive medication in black and white medicaid recipients. Hypertension 39:179–183
Koike K, Ikezumi Y, Tsuboi N, Kanzaki G, Haruhara K, Okabayashi Y, Sasaki T, Ogura M, Saitoh A, Yokoo T (2017) Glomerular density and volumen in renal biopsy specimens of children with proteinuria relative to preterm birth and gestational age. Clin J Am Soc Nephrol 12:585–590
Black MJ, Sutherland MR, Gubhaju L, Kent AL, Dahlstrom JE, Moore L (2013) When birth comes early: effects on nephrogenesis. Nephrology 18:180–182
Sutherland MR, Gubhaju L, Yoder BA, Stahlman MT, Black MJ (2009) The effects of postnatal retinoic acid administration on nephron endowment in the preterm baboon kidney. Pediatr Res 65:397–402
Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, Hoy WE, Bertram JF, Black MJ (2011) Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol 22:1365–1374
Coca SG, Singanamala S, Parikh CR (2012) Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81:442–448
See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, Toussaint ND, Bellomo R (2019) Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 95:160–172
Chawla LS, Eggers PW, Star RA, Kimmel PL (2014) Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371:58–66
Basile DP, Bonventre JV, Mehta R, Nangaku M, Unwin R, Rosner MH, Kellum JA, Ronco C (2016) Progression after AKI: understanding maladaptive repair processes to predict and idnetify therapeutic treatments. J Am Soc Nephrol 27:687–697
Greenberg JH, Coca SG, Parikh CR (2014) Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol 15:184
Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG (2012) Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 59:523–530
Hessey E, Perreault S, Dorais M, Roy L, Zappitelli M (2019) Acute kidney injury in critically ill children and subsequent chronic kidney disease. Can J Kidney Health Dis 6:2054358119880188
Menon S, Kirkendall ES, Nguyen H, Goldstein SL (2014) Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr 165:522–527
Hukriede N, Soranno D, Starr M, Sander V, Perreau T, Yuen P, Siskind L, Hutchens M, Davidson A, Burmeister D, Faubel S, de Caestecker M (2022) Experimental models of acute kidney injury for translational research. Nat Rev Nephrol 18:277–293
Chevalier RL, Goyal S, Wolstenholme JT, Thornhill BA (1998) Obstructive nephropathy in the neonatal rat is attenuated by epidermal growth factor. Kidney Int 54:38–47
Liberio BM, Seedorf G, Soranno DE, Montford JR, Faubel SG, Hernandez A, Abman SH, Gien J (2023) Acute kidney injury decreases pulmonary vascular growth and alveolarization in neonatal rat pups. Pediatr Res. https://doi.org/10.1038/s41390-023-02625-y
Tsujimoto H, Kasahara T, Sueta SI, Araoka T, Sakamoto S, Okada C, Mae SI, Nakajima T, Okamoto N, Taura D, Nasu M, Shimizu T, Ryosaka M, Li Z, Sone M, Ikeya M, Watanabe A, Osafune K (2020) A modular differentiation system maps multiple human kidney lineages from pluripotent stem cells. Cell Rep 31:107476
Bairey Merz CN, Dember LM, Ingelfinger JR, Vinson A, Neugarten J, Sandberg KL, Sullivan JC, Maric-Bilkan C, Rankin TL, Kimmel PL, Star RA, participants of the National Institute of Diabetes and Digestive and Kidney Diseases Workshop on “Sex and the Kidneys” (2019) Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol 15(12):776–783. https://doi.org/10.1038/s41581-019-0208-6
Neugarten J, Golestaneh L (2019) Influence of sex on the progression of chronic kidney disease. Mayo Clin Proc 94:1339–1356
Neugarten J, Golestaneh L (2018) Female sex reduces the risk of hospital-associated acute kidney injury: a meta-analysis. BMC Nephrol 19:314
Neugarten J, Golestaneh L (2016) The effect of gender on aminoglycoside-associated nephrotoxicity. Clin Nephrol 86:183–189
Neugarten J, Golestaneh L (2013) Gender and the prevalence and progression of renal disease. Adv Chronic Kidney Dis 20:390–395
Bairey Merz CN, Dember LM, Ingelfinger JR, Vinson A, Neugarten J, Sandberg KL, Sullivan JC, Maric-Bilkan C, Rankin TL, Kimmel PL, Star RA (2019) Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol 15:776–783
Faubel S, Edelstein CL (2016) Mechanisms and mediators of lung injury after acute kidney injury. Nat Rev Nephrol 12:48–60
Starr MC, Boohaker L, Eldredge LC, Menon S, Griffin R, Mayock DE, Li L, Askenazi D, Hingorani S (2020) Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks’ gestation. Am J Perinatol 37:341–348
Sarkar S, Askenazi DJ, Jordan BK, Bhagat I, Bapuraj JR, Dechert RE, Selewski DT (2014) Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr Res 75:431–435
Soranno DE, Baker P 2nd, Kirkbride-Romeo L, Wennersten SA, Ding K, Keith B, Cavasin MA, Altmann C, Bagchi RA, Haefner KR, Montford J, Gist KM, Vergnes L, Reue K, He Z, Elajaili H, Okamura K, Nozik E, McKinsey TA, Faubel S (2022) Female and male mice have differential longterm cardiorenal outcomes following a matched degree of ischemia-reperfusion acute kidney injury. Sci Rep 12:643
Soranno DE, Kirkbride-Romeo L, Wennersten SA, Ding K, Cavasin MA, Baker P, Altmann C, Bagchi RA, Haefner KR, Steinkuhler C, Montford JR, Keith B, Gist KM, McKinsey TA, Faubel S (2021) Acute kidney injury results in long-term diastolic dysfunction that is prevented by histone deacetylase inhibition. JACC Basic Transl Sci 6:119–133
Fitzgerald JC, Basu RK, Akcan-Arikan A, Izquierdo LM, Pineres Olave BE, Hassinger AB, Szczepanska M, Deep A, Williams D, Sapru A, Roy JA, Nadkarni VM, Thomas NJ, Weiss SL, Furth S; Sepsis PRevalence, OUtcomes, and Therapies Study Investigators and Pediatric Acute Lung Injury and Sepsis Investigators Network (2016) Acute kidney injury in pediatric severe sepsis: an independent risk factor for death and new disability. Crit Care Med 44(12):2241–2250. https://doi.org/10.1097/CCM.0000000000002007
Starr MC, Banks R, Reeder RW, Fitzgerald JC, Pollack MM, Meert KL, McQuillen PS, Mourani PM, Chima RS, Sorenson S, Varni JW, Hingorani S, Zimmerman JJ, Life After Pediatric Sepsis Evaluation (LAPSE) (2020) Severe acute kidney injury is associated with increased risk of death and new morbidity after pediatric septic shock. Pediatr Crit Care Med 21:e686–e695
Smith M, Bell C, Vega MW, Tufan Pekkucuksen N, Loftis L, McPherson M, Graf J, Akcan Arikan A (2022) Patient-centered outcomes in pediatric continuous kidney replacement therapy: new morbidity and worsened functional status in survivors. Pediatr Nephrol 37:189–197
Desanti De Oliveira B, Xu K, Shen TH, Callahan M, Kiryluk K, D’Agati VD, Tatonetti NP, Barasch J, Devarajan P (2019) Molecular nephrology: types of acute tubular injury. Nat Rev Nephrol 15:599–612
de Caestecker M, Humphreys BD, Liu KD, Fissell WH, Cerda J, Nolin TD, Askenazi D, Mour G, Harrell FE Jr, Pullen N, Okusa MD, Faubel S; ASN AKI Advisory Group (2015) Bridging translation by improving preclinical study design in AKI. J Am Soc Nephrol 26:2905–2916
Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R (2019) Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16:255–262
Zeng Z, Huang B, Parvez RK, Li Y, Chen J, Vonk AC, Thornton ME, Patel T, Rutledge EA, Kim AD, Yu J, Grubbs BH, McMahon JA, Pastor-Soler NM, Hallows KR, McMahon AP, Li Z (2021) Generation of patterned kidney organoids that recapitulate the adult kidney collecting duct system from expandable ureteric bud progenitors. Nat Commun 12:3641
Lawlor KT, Vanslambrouck JM, Higgins JW, Chambon A, Bishard K, Arndt D, Er PX, Wilson SB, Howden SE, Tan KS, Li F, Hale LJ, Shepherd B, Pentoney S, Presnell SC, Chen AE, Little MH (2021) Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat Mater 20:260–271
Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M, Leopoldi A, Garreta E, Hurtado Del Pozo C, Prosper F, Romero JP, Wirnsberger G, Zhang H, Slutsky AS, Conder R, Montserrat N, Mirazimi A, Penninger JM (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181(4):905–913.e7. https://doi.org/10.1016/j.cell.2020.04.004
Wysocki J, Ye M, Hassler L, Gupta AK, Wang Y, Nicoleascu V, Randall G, Wertheim JA, Batlle D (2021) A novel soluble ACE2 variant with prolonged duration of action neutralizes SARS-CoV-2 infection in human kidney organoids. J Am Soc Nephrol 32:795–803
Ooms A, Calandrini C, de Krijger RR, Drost J (2020) Organoid models of childhood kidney tumours. Nat Rev Urol 17:311–313
Devarajan P (2006) Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17:1503–1520
Li L, Okusa MD (2006) Blocking the immune response in ischemic acute kidney injury: the role of adenosine 2A agonists. Nat Clin Pract Nephrol 2:432–444
Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA (2001) Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 60:2118–2128
Heemskerk S, Masereeuw R, Russel FG, Pickkers P (2009) Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nat Rev Nephrol 5:629–640
Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, Brafman A, Faerman A, Atkinson SJ, Thompson JD, Kalinski H, Skaliter R, Erlich S, Feinstein E (2009) siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol 20:1754–1764
Zhang XH, Li ML, Wang B, Guo MX, Zhu RM (2014) Caspase-1 inhibition alleviates acute renal injury in rats with severe acute pancreatitis. World J Gastroenterol 20:10457–10463
Guerrero-Hue M, Rubio-Navarro A, Sevillano A, Yuste C, Gutierrez E, Palomino-Antolin A, Roman E, Praga M, Egido J, Moreno JA (2018) Adverse effects of the renal accumulation of haem proteins. Novel therapeutic approaches. Nefrologia (Engl Ed) 38:13–26
Peng J, Li X, Zhang D, Chen JK, Su Y, Smith SB, Dong Z (2015) Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int 87:137–150
Kwak J, Kim JH, Jang HN, Jung MH, Cho HS, Chang SH, Kim HJ (2020) Erythropoietin ameliorates ischemia/reperfusion-induced acute kidney injury via inflammasome suppression in mice. Int J Mol Sci 21:3453
Kawaida K, Matsumoto K, Shimazu H, Nakamura T (1994) Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci U S A 91:4357–4361
Jones NK, Stewart K, Czopek A, Menzies RI, Thomson A, Moran CM, Cairns C, Conway BR, Denby L, Livingstone DEW, Wiseman J, Hadoke PW, Webb DJ, Dhaun N, Dear JW, Mullins JJ, Bailey MA (2020) Endothelin-1 mediates the systemic and renal hemodynamic effects of GPR81 activation. Hypertension 75:1213–1222
Packialakshmi B, Stewart IJ, Burmeister DM, Chung KK, Zhou X (2020) Large animal models for translational research in acute kidney injury. Ren Fail 42:1042–1058
Jenik AG, Ceriani Cernadas JM, Gorenstein A, Ramirez JA, Vain N, Armadans M, Ferraris JR (2000) A randomized, double-blind, placebo-controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics 105:E45
Raina A, Pandita A, Harish R, Yachha M, Jamwal A (2016) Treating perinatal asphyxia with theophylline at birth helps to reduce the severity of renal dysfunction in term neonates. Acta Paediatr 105:e448-451
Harer MW, Askenazi DJ, Boohaker LJ, Carmody JB, Griffin RL, Guillet R, Selewski DT, Swanson JR, Charlton JR, Neonatal Kidney Collaborative (NKC) (2018) Association between early caffeine citrate administration and risk of acute kidney injury in preterm neonates: results from the AWAKEN study. JAMA Pediatr 172:e180322
Lange C, Togel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C (2005) Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int 68:1613–1617
Togel FE, Westenfelder C (2012) Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis 60:1012–1022
Huang S, Li Y, Wang X, Ma X, Zhang X (2017) Injectable co-gels of collagen and decellularized vascular matrix improve MSC-based therapy for acute kidney injury. J Biomater Sci Polym Ed 28:2186–2195
Tsurkan MV, Hauser PV, Zieris A, Carvalhosa R, Bussolati B, Freudenberg U, Camussi G, Werner C (2013) Growth factor delivery from hydrogel particle aggregates to promote tubular regeneration after acute kidney injury. J Control Release 167:248–255
Soranno DE, Lu HD, Weber HM, Rai R, Burdick JA (2014) Immunotherapy with injectable hydrogels to treat obstructive nephropathy. J Biomed Mater Res A 102:2173–2180
Basu J, Genheimer CW, Rivera EA, Payne R, Mihalko K, Guthrie K, Bruce AT, Robbins N, McCoy D, Sangha N, Ilagan R, Knight T, Spencer T, Wagner BJ, Jayo MJ, Jain D, Ludlow JW, Halberstadt C (2011) Functional evaluation of primary renal cell/biomaterial neo-kidney augment prototypes for renal tissue engineering. Cell Transplant 20:1771–1790
Rodell CB, Rai R, Faubel S, Burdick JA, Soranno DE (2015) Local immunotherapy via delivery of interleukin-10 and transforming growth factor beta antagonist for treatment of chronic kidney disease. J Control Release 206:131–139
Soranno DE, Rodell CB, Altmann C, Duplantis J, Andres-Hernando A, Burdick JA, Faubel S (2016) Delivery of interleukin-10 via injectable hydrogels improves renal outcomes and reduces systemic inflammation following ischemic acute kidney injury in mice. Am J Physiol Renal Physiol 311:F362-372
Jansen K, Schuurmans CCL, Jansen J, Masereeuw R, Vermonden T (2017) Hydrogel-based cell therapies for kidney regeneration: current trends in biofabrication and in vivo repair. Curr Pharm Des 23:3845–3857
Xu Q, He C, Xiao C, Chen X (2016) Reactive oxygen species (ROS) responsive polymers for biomedical applications. Macromol Biosci 16:635–646
Benoit SW, Devarajan P (2018) Acute kidney injury: emerging pharmacotherapies in current clinical trials. Pediatr Nephrol 33:779–787
Okusa MD, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative XIII Workgroup (2016) Therapeutic targets of human AKI: harmonizing human and animal AKI. J Am Soc Nephrol 27(1):44–48. https://doi.org/10.1681/ASN.2015030233
Zuk A, Palevsky PM, Fried L, Harrell FE Jr, Khan S, McKay DB, Devey L, Chawla L, de Caestecker M, Kaufman JS, Thompson BT, Agarwal A, Greene T, Okusa MD, Bonventre JV, Dember LM, Liu KD, Humphreys BD, Gossett D, Xie Y, Norton JM, Kimmel PL, Star RA (2018) Overcoming translational barriers in acute kidney injury: a report from an NIDDK workshop. Clin J Am Soc Nephrol 13:1113–1123
Freeman RB, Huang W (2014) Collaboration: strength in diversity. Nature 513:305
Galinsky AD, Todd AR, Homan AC, Phillips KW, Apfelbaum EP, Sasaki SJ, Richeson JA, Olayon JB, Maddux WW (2015) Maximizing the gains and minimizing the pains of diversity: a policy perspective. Perspect Psychol Sci 10:742–748
Kashani K, Awdishu L, Bagshaw S, Barreto E, Granado R, Evans B, Forni L, Ghosh E, Goldstein S, Kane-Gill S, Kooola J, Koyner J, Liu M, Murugan R, Nadkarni G, Neyra J, Ninan J, Ostermann M, Pannu N, Rashidi P, Ronco C, Rosner M, Selby SB, Singh K, Soranno D, Sutherland S, Bihorac A, Mehta R (2023) Digital health and acute kidney injury: summary of recommendations from the 27th Acute Disease Quality Initiative Conference. Nat Rev Nephrol. https://doi.org/10.1038/s41581-023-00744-7
Hanson CS, Gutman T, Craig JC, Bernays S, Raman G, Zhang Y, James LJ, Ralph AF, Ju A, Manera KE, Teixeira-Pinto A, Viecelli AK, Alexander SI, Blydt-Hansen TD, Dionne J, McTaggart S, Michael M, Walker A, Carter S, Wenderfer SE, Winkelmayer WC, Bockenhauer D, Dart A, Eddy AA, Furth SL, Gipson DS, Goldstein SL, Groothoff J, Samuel S, Sinha A, Webb NJA, Yap HK, Zappitelli M, Currier H, Tong A (2019) Identifying important outcomes for young people with CKD and their caregivers: a nominal group technique study. Am J Kidney Dis 74:82–94
Lunyera J, Kilonzo K, Lewington A, Yeates K, Finkelstein FO (2016) Acute kidney injury in low-resource settings: barriers to diagnosis, awareness, and treatment and strategies to overcome these barriers. Am J Kidney Dis 67:834–840
AlShebli BK, Rahwan T, Woon WL (2018) The preeminence of ethnic diversity in scientific collaboration. Nat Commun 9:5163
Soranno DE, Simon TD, Bora S, Lohr JL, Bagga B, Carroll K, Daniels SR, Davis SD, Fernandez Y, Garcia E, Orange JS, Overholser B, Sedano S, Tarini BA, White MJ, Spector ND (2023) Justice, equity, diversity, and inclusion in the pediatric faculty research workforce: call to action. Pediatrics 152:e2022060841. https://doi.org/10.1542/peds.2022-060841
US Government Accountability Office (2018) National Institutes of Health: Kidney Disease Research Funding and Priority Setting, GAO-17-121. https://www.gao.gov/assets/gao-17-121.pdf
Luyckx VA, Tonelli M, Stanifer JW (2018) The global burden of kidney disease and the sustainable development goals. Bull World Health Organ 96:414-422D
Askenazi DJ, Heagerty PJ, Schmicker RH, Brophy P, Juul SE, Goldstein SL, Hingorani S (2021) The impact of erythropoietin on short and long-term kidney-related outcomes in extremely low gestational age neonates. Results of a multi-center double-blind placebo-controlled randomized clinical trial. J Pediatr 232:65-72.e7
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Starr, M.C., Barreto, E., Charlton, J. et al. Advances in pediatric acute kidney injury pathobiology: a report from the 26th Acute Disease Quality Initiative (ADQI) conference. Pediatr Nephrol 39, 941–953 (2024). https://doi.org/10.1007/s00467-023-06154-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-023-06154-y