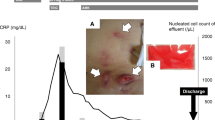
Abstract
Background
Peritonitis is an important complication and cause of morbidity in patients undergoing peritoneal dialysis (PD). Corynebacterium species, often considered skin and mucosal contaminants, are a rare cause of PD-associated peritonitis and have been acknowledged in published guidelines for the diagnosis and treatment of PD peritonitis only over the last decade.
Case-Diagnosis/Treatment
We present two children with difficult-to-treat episodes of PD peritonitis due to Corynebacterium amycolatum. Episodes were associated with fever, abdominal pain and cloudy dialysate, high dialysate polymorphonuclear leukocyte counts, and elevated serum C-reactive protein and procalcitonin concentrations. Symptoms persisted beyond 5 days in 4 of 5 peritonitis episodes, and peritonitis relapsed despite in vitro sensitivity of the bacterial isolates to guideline-recommended antibiotics. C. amycolatum was cultured from the PD catheter tip despite 4 weeks of intraperitoneal glycopeptide therapy and clinical peritonitis resolution suggestive of efficient biofilm formation. Our systematic literature search identified three previous (adult) case descriptions of C. amycolatum peritonitis, all with repeat episodes by the same organism. The incidence of C. amycolatum as a cause of PD peritonitis has not yet been established but is likely underreported due to challenges in species differentiation.
Conclusions
C. amycolatum is a rarely identified cause of refractory and/or relapsing PD peritonitis. Species differentiation of non-diphtheriae Corynebacterium isolates is critical, and prolonged antibiotic treatment, preferably with a glycopeptide antibiotic, is recommended, with a low threshold for PD catheter change or removal in case of repeat peritonitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peritonitis represents an important complication in patients receiving chronic peritoneal dialysis (PD) leading to hospitalization, peritoneal membrane failure, PD catheter loss, and change of dialysis modality [1]. Corynebacteria are Gram-positive, facultatively anaerobic, nonsporulating, generally non-motile rods [2]. Non-diphtheriae (coryneform) Corynebacterium species belong to the physiological flora of human skin and mucous membranes [2]. Species level differentiation can be challenging, especially between C. striatum, C. amycolatum, and C. xerosis [2, 3]. C. amycolatum, a non-lipophilic Corynebacterium that lacks detectable mycolic acids found in the remainder of Corynebacteria [2], is now considered a common opportunistic pathogen in humans [2, 4]. Antibiotic sensitivity of clinical isolates is variable, but all strains are susceptible to glycopeptides [2].
To date, only three cases of PD peritonitis due to C. amycolatum have been reported, all in adults [5,6,7]. Here, we present two children with several episodes of C. amycolatum peritonitis highlighting therapeutic challenges and the growing importance of this organism.
Peritonitis definitions are from the current International Society of Peritoneal Dialysis (ISPD) guidelines [8]. Peritoneal effluent for cell count and differentiation and for microbial culture was obtained according to standard recommendations [8, 9]. “Day 1” is defined as the date of clinical diagnosis, when effluent dialysate was sent for microscopy and culture. Empirical peritonitis treatment consisted of intraperitoneal (IP) cefepime or the combination of IP ceftazidime and vancomycin [9], in addition to heparin 250–500 units/L and oral fluconazole.
Case 1
The patient was a 4-year-old boy with CKD stage 5D secondary to congenital bilateral kidney hypodysplasia. He commenced chronic automated PD (APD) at the age of 2 years. A first peritonitis due to Acinetobacter baumannii 1 year after PD initiation was successfully treated with IP antibiotics. A year later, he presented with intermittent abdominal pain over 2 days and cloudy dialysate effluent. The peritoneal effluent white blood cell (WBC) count was 308/µL (36.1% neutrophils). Gram stain and culture of the effluent dialysis remained negative after 5 days of incubation. Peripheral WBC was 13.4 × 109/L and C-reactive protein (CRP) 54.9 mg/L (normal < 5 mg/L). He was discharged and observed closely. A week later, he presented with scrotal pain, sluggish peritoneal drainage, and peripheral edema. Ultrasound showed a strangulated inguinal hernia prompting emergency herniotomy. Perioperatively, he received a dose of intravenous (IV) ceftriaxone. He resumed PD 2 days after surgery but returned the same evening with fever and severe, diffuse abdominal tenderness. PD catheter tunnel/exit site and herniotomy incision were intact. Microscopy of the dialysate revealed 482 WBC/µL. Serum CRP (59.1 mg/L) and procalcitonin (3.67 ng/mL, normal < 0.05 ng/mL) were elevated, and IP treatment with cefepime was started. Pre-treatment dialysate effluent (50 mL) was used to inoculate aerobic and anaerobic blood culture bottles for enrichment (5 mL each). The remainder of the effluent was centrifuged, and the sediment directly plated on various media, including chocolate and fastidious anaerobic agar. The dialysate showed Gram-positive rods with numerous WBC. Blood culture bottles flagged positive after 24 h of incubation. Subcultures, plated directly on blood and chocolate agar, yielded growth of small gray, flat colonies after 48 h of incubation. They were identified as C. amycolatum using the VITEK 2 ANC (Anaerobic and Corynebacterium) Identification Card (BioMérieux), confirmed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF; National Reference Laboratory, UAE). Isolates were in vitro susceptible to penicillin, cephalosporins, clindamycin, and glycopeptides and resistant to trimethoprim/sulfamethoxazole (TMP/SMX) using Clinical and Laboratory Standards Institute (CLSI M45) guidelines [10]. Repeat dialysate effluent cultures on days 2 and 9 were sterile, and antibiotic treatment was discontinued after a total of 3 weeks.
The patient returned 2 days later with new effluent drain pain and dialysate pleocytosis (2,844 WBC/µL, 54% neutrophils). Treatment was restarted with IP cefepime. Effluent culture again yielded C. amycolatum. Because the effluent failed to clear, IP teicoplanin was added for a total antibiotic treatment duration of 4 weeks. Repeat effluent analyses were normal, and cultures remained negative following the completion of antimicrobial therapy (Table 1).
Seven months later, the patient presented again with cloudy dialysate and fever. The effluent showed 1674/µL WBC (41% neutrophils), and empiric treatment was started with IP cefepime. C. amycolatum was isolated and teicoplanin added. Swabs from PD exit site and groin (but not from nose and throat) were positive for C. amycolatum with identical antibiotic sensitivities.
A week after treatment completion, the child received a deceased donor kidney transplant with standard perioperative cefazolin prophylaxis. The PD catheter was removed during the transplant surgery, and C. amycolatum was grown from its tip. There were no signs of peritonitis or bacteremia post-transplant (Table 1).
Case 2
The patient was a 5-year-old girl with CKD stage 5D secondary to congenital nephrotic syndrome, APD since age 2 years. She had preceding peritonitis episodes due to Staphylococcus epidermidis and Streptococcus viridans. At the index episode, she presented with fever, vomiting, lower abdominal pain, and poor oral intake for the past 2 days. Clinical examination showed fever of 38.7 °C and mild abdominal tenderness. Peripheral WBC was normal, yet CRP and procalcitonin were significantly elevated. Dialysate effluent revealed 2161 WBC/μL (85% neutrophils), and peritonitis treatment was started with IP ceftazidime and vancomycin. She remained febrile over the next 48 h, accompanied by multiple hypotensive episodes and rising serum CRP and procalcitonin concentrations prompting fluid boluses, transfer to the pediatric intensive care unit due to suspected sepsis (days 3–5), and addition of IV meropenem. The latter was discontinued when the effluent culture result became available: C. amycolatum, in vitro sensitive to erythromycin, gentamicin, TMP/SMX, vancomycin; resistant to clindamycin. Oral TMP/SMX was added on day 10 because of persistent fever and high effluent WBC. The patient was discharged on day 11 against medical advice. IP and oral antibiotics were continued for a total of 3 weeks (Table 1).
She was readmitted with severe abdominal pain 3 weeks after discontinuation of the antibiotics, with 225 WBC/μL effluent (94% neutrophils). IP ceftazidime and vancomycin were restarted and continued at home. The dialysate culture remained negative. She returned on day 9 of the peritonitis relapse due to persistent abdominal pain, associated with rising inflammatory markers (Table 1). Oral TMP/SMX was again added without clinical improvement, prompting PD catheter removal and transfer to HD.
Literature review
In a systemic PubMed and Google Scholar search without language restriction, we identified three previously published (adult) cases of C. amycolatum PD peritonitis (Table 2). In the first reported case of a 65-year-old woman, the isolate was sensitive in vitro to all antibiotics used. Peritonitis only resolved after switching to IP vancomycin. Repeat peritonitis 3.5 months after treatment completion led to PD catheter removal and IV vancomycin administration, followed by successful PD re-initiation. Identity of both C. amycolatum isolates was demonstrated by pulsed-field gel electrophoresis [5]. Sonmezer et al. described a 55-year-old patient who developed C. amycolatum peritonitis associated with increased peripheral WBC count and CRP [6]. Effluent dialysate cleared after switching from IP cefazolin and gentamicin to IP vancomycin, yet she returned with turbid effluent five days after discharge. The infection resolved following combined IP and IV vancomycin administration (see Table 2). The third patient was identified in a study evaluating the utility of a taurolidine/citrate/urokinase PD catheter “lock” in patients with frequent peritonitis. The treatment protocol consisted of IP vancomycin and an aminoglycoside for 14–21 days. One of six enrolled patients had multiple episodes of C. amycolatum peritonitis, but further clinical details are missing [7]. Ubaldi et al. assessed the frequency of Corynebacterium isolates in a medical microbiology laboratory over a 3-year period [11]. Corynebacterium was cultured from 31 PD catheter exit sites and six PD fluid samples. Four and two isolates, respectively, were identified as C. amycolatum, without demographic and clinical data.
Discussion
C. amycolatum PD peritonitis has not yet been reported in the pediatric age group. Although rare, the true incidence may be greater than suggested by the current literature [11]. Species differentiation of non-diphtheriae Corynebacterium strains is technically demanding [2, 12], leading to delayed resulting and genus-level reporting only. For example, a recent registry study comprising 11,122 PD peritonitis episodes in adults attributed 162 episodes (1.5% of all peritonitis cases) to non-diphtheriae Corynebacteria. The authors found no difference in relevant outcome parameters, such as clearing of infection, PD catheter survival, or peritonitis-related death between episodes due to Corynebacteria or other Gram-positive organisms [13]. In contrast, an earlier study from Hong Kong reported increased rates of repeat peritonitis due to non-diphtheriae Corynebacteria [14]. Neither publication provided species level identification, which may have obscured differences in species-related outcomes. Species identification has clinical importance, allowing to separate chronic from de novo infections and to adjust antibiotic treatment or opt for PD catheter removal if the same strain is isolated repeatedly.
Based on these cases, a picture emerges of C. amycolatum as a cause of PD peritonitis with notable, occasionally severe systemic inflammation that tends to be refractory to conventional, guideline-based treatment and prone to repeat episodes even after prolonged periods of quiescence. Interestingly, effluent cultures became promptly negative after initiation of IP antibiotic therapy in our patients, yet dialysate pleocytosis and abdominal discomfort persisted in four of the five (refractory) episodes [8] (Table 1). Both patients experienced “relapsing” peritonitides, defined as occurrence within 4 weeks of treatment completion, even in the absence of bacterial growth [8] (Table 1, episode 2b). It is not primary antimicrobial resistance but PD catheter biofilm formation that appears to complicate conventional treatment of C. amycolatum peritonitis [7, 15]. Possible targets for research are the development of biofilm-disruptive therapies, understanding bacterial and host factors that facilitate biofilm formation, and strategies for its prevention.
References
Sethna CB, Bryant K, Munshi R, Warady BA, Richardson T, Lawlor J, Newland JG, Neu A, SCOPE Investigators (2016) Risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE collaborative. Clin J Am Soc Nephrol 11:1590–1596
Bernard KA (2019) Coryneform gram-positive rods. In: Carroll KC, Pfaller MA, Landry ML, McAdam AJ, Patel R, Richter SS, Warnock DW (eds) Manual of clinical microbiology. ASM Press, Washington, DC, pp 488–534
Zasada AA, Mosiej E (2018) Contemporary microbiology and identification of Corynebacteria spp. causing infections in human. Lett Appl Microbiol 66:472–483
Carvalho RV, Lima F, Santos CSD, Souza MC, Silva RSD, Mattos-Guaraldi AL (2018) Central venous catheter-related infections caused by Corynebacterium amycolatum and other multiresistant non-diphtherial corynebacteria in paediatric oncology patients. Braz J Infect Dis 22:347–351
Chiu YL, Wu VC, Wun KD, Hsueh PR (2005) Recurrent peritonitis caused by Corynebacterium amycolatum in a patient undergoing continuous ambulatory peritoneal dialysis. Clin Nephrol 63:241–242
Sonmezer MC, Ertem GT, Yetkin MA, Eda Yıldız, Oral B (2013) Relapsing peritonitis caused by Corynebacterium amycolatum in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. Int J Infect Control 9:(1). https://doi.org/10.3396/ijic.v9i1.010.13
Sosa Barrios RH, Alvarez Nadal M, Burguera Vion V, Campillo Trapero C, Lopez Melero E, Fernandez Lucas M, Rivera Gorrin ME (2021) Relapsing peritonitis and taurolidine peritoneal catheter lock: one center experience. J Vasc Access 22:261–265
Li PK, Chow KM, Cho Y, Fan S, Figueiredo AE, Harris T, Kanjanabuch T, Kim YL, Madero M, Malyszko J, Mehrotra R, Okpechi IG, Perl J, Piraino B, Runnegar N, Teitelbaum I, Wong JK, Yu X, Johnson DW (2022) ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int 42:110–153
Warady BA, Bakkaloglu S, Newland J, Cantwell M, Verrina E, Neu A, Chadha V, Yap HK, Schaefer F (2012) Consensus guidelines for the prevention and treatment of catheter-related infections and peritonitis in pediatric patients receiving peritoneal dialysis: 2012 update. Perit Dial Int 32(Suppl 2):S32–S86
CLSI (2016) Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria, 3rd ed, CLSI Guideline M45. Clinical and Laboratory Standards Institute, Wayne, PA
Ubaldi MDAM, Medori MC, Crotti D (2004) Corinebatteri e corineformi: ruolo eziologico in pazienti ospedalizzati e fenotipi di resistenza nel corso di 3 anni di osservazione [Coryneform bacteria: their clinical significance and resistance patterns during a three-year study]. Infez Med 12:126–131
Santos CS, Ramos JN, Vieira VV, Pinheiro CS, Meyer R, Alcantara-Neves NM, Ramos RT, Silva A, Hirata R Jr, Felicori L, de Alegria Puig CR, Navas J, Azevedo V, Mattos-Guaraldi AL, Pacheco LGC (2017) Efficient differentiation of Corynebacterium striatum, Corynebacterium amycolatum and Corynebacterium xerosis clinical isolates by multiplex PCR using novel species-specific primers. J Microbiol Methods 142:33–35
Htay H, Cho Y, Pascoe EM, Darssan D, Hawley C, Clayton PA, Borlace M, Badve SV, Sud K, Boudville N, McDonald SP, Johnson DW (2017) Outcomes of Corynebacterium Peritonitis: A Multicenter Registry Analysis. Perit Dial Int 37:619–626
Szeto CC, Chow KM, Chung KY, Kwan BC, Leung CB, Li PK (2005) The clinical course of peritoneal dialysis-related peritonitis caused by Corynebacterium species. Nephrol Dial Transplant 20:2793–2796
Olender A, Bogut A, Magrys A, Krol-Turminska K (2018) A Novel Approach to Study the Effect of Ciprofloxacin on Biofilms of Corynebacterium spp. Using Confocal Laser Scanning Microscopy. Pol J Microbiol 67:431–440
Acknowledgements
Bacterial cultures were performed at the Al Jalila Children’s Hospital and the Dubai Hospital clinical microbiology laboratories.
Author information
Authors and Affiliations
Contributions
SMH collected and summarized clinical data and wrote the first draft of the manuscript. HY collected and summarized clinic data. RL was responsible for laboratory testing and reviewed and edited the manuscript. MB initiated and supervised the study, confirmed the clinical and laboratory data, and wrote the final version of the manuscript. All authors were involved in the care of the described patients and edited and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Written informed consent for publication was provided by the participants’ legal guardians.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Habeeb, S.M., Yamin, H., Simkova, E. et al. Relapsing and refractory peritoneal dialysis peritonitis caused by Corynebacterium amycolatum. Pediatr Nephrol 38, 1687–1692 (2023). https://doi.org/10.1007/s00467-022-05801-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05801-0