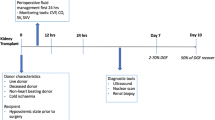
Abstract
Background
Intravenous fluid administration is an essential part of perioperative care for children receiving a kidney transplant. There is a paucity of evidence to guide optimal perioperative fluid management. This study aimed to identify the volume of perioperative fluids administered across 5 UK paediatric kidney transplant centres and explore associations between fluid volume administered, graft function, and fluid-related adverse events.
Methods
Data were collected from five UK paediatric kidney transplant centres on perioperative fluid volumes administered, and incidence of pulmonary oedema, systemic hypertension, and requirement for intensive care support. Children < 18 years of age who received a kidney-only transplant between 1st January 2020 and 31st December 2021 were included.
Results
Complete data from 102 children were analysed. The median total volume of fluid administered in 72 h was 377 ml/kg (IQR 149 ml/kg) with a high degree of variability. A negative relationship between total fluid volume administered and day 7 eGFR was noted (p < 0.001). Association between urine volume post-transplant and day 7 eGFR was also negative (p < 0.001). Adverse events were frequent but no significant difference was found in the fluid volume administered to those who developed an adverse event, vs those who did not.
Conclusions
This study describes a high degree of variability in perioperative fluid volumes administered to children receiving kidney transplants. Both fluid volume and urine output were negatively associated with short-term graft function. These data contrast traditional interpretation of high urine output as a marker of graft health, and highlight the need for prospective clinical trials to optimise perioperative fluid administration for this group.
Graphical Abstract
A higher resolution version of the Graphical abstract is available as Supplementary information

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kidney transplantation is the treatment of choice for children with kidney failure [1]. Kidney transplant innovations have improved the outcomes in children by addressing many factors such as immunosuppression, HLA matching [2], and controlling ischaemia time [3]. However, other modifiable factors have not received as much attention in clinical research. Fluid management is one such factor. In adult transplant recipients, judicious use of fluids was found to be beneficial [4, 5], but there is a high degree of variability in fluid management strategies used, with no consensus on the most advantageous approach [6]. There is also a paucity of high-quality evidence to guide optimal perioperative fluid management in children receiving kidney transplants.
Ensuring adequate perfusion of a transplant kidney is important as intravascular volume depletion brings risk of delayed graft function and acute tubular injury. Delayed graft function in adult recipients is associated with reduced graft survival [7]. Whilst delayed graft function is multifactorial in aetiology, it is exacerbated by poor perfusion with resultant acute tubular injury or kidney arterial thrombosis. The denervation of a graft kidney in the process of transplantation hampers its capacity to regulate blood flow, and as a result, ischaemia and functional impairment may ensue more easily in response to poor perfusion when compared to healthy native kidneys [8].
Whilst inadequate fluid administration may impair graft perfusion, superfluous fluid intake increases the risk of fluid overload with consequential respiratory compromise and hypertension. Respiratory compromise may result in unexpected paediatric intensive care admission and the need for ventilatory support. Systemic hypertension may result in end-organ damage to sensitive vascular beds in the brain and lungs for example. It lies with the healthcare professional team to determine the appropriate volume of fluid to administer to children following kidney transplant.
Current fluid management practices in the immediate postoperative phase following kidney transplantation in children have not been described in the literature. Differing strategies are known to be used, including insensible losses plus ml for ml replacement of urine output and fixed target fluid administration. This study aims to describe the volumes of fluid administered perioperatively across 5 large UK paediatric kidney transplant centres and explore if there are associations between the fluid volume administered with overall graft health or the incidence of adverse events including pulmonary oedema, hypertension, and requirement for intensive care support.
Method
Study design
Anonymised perioperative data for children under 18 years of age transplanted between 1 January 2020 and 31 December 2021 in five UK paediatric nephrology transplant centres was collated retrospectively. Those with incomplete data regarding fluid intake and urine output in the first 3 postoperative days were excluded from the analysis.
Data were fully anonymised at individual hospital sites and ethical principles adhered to throughout. The study received local ethical approval, and the requirement for individual participant consent was waived.
Study procedures
Participants were identified by a lead clinician at each hospital and included all children who had received a kidney transplant between 1st January 2020 and 31st December 2021. Anonymised data were collected by the lead clinician at each hospital via a standardised spreadsheet.
Systemic hypertension was defined as systolic blood pressure above the 95th centile for age and height on two or more consecutive readings [9]. Pulmonary oedema was defined as radiographic evidence of interstitial lung fluid with associated symptoms or signs. Delayed graft function was defined by the requirement for kidney replacement therapy within the first 7 days post-transplant.
Statistical analysis
Continuous data are summarised as median (interquartile range) or median (range). Non-parametric tests (Wilcoxon’s signed-rank and Mann–Whitney U) were used for statistical comparisons. A P value < 0.05 was considered statistically significant.
Pre-specified analyses included a comparison of fluid volumes received for children < 20 kg vs ≥ 20 kg bodyweight, relationship between fluid volume administered and day 7 eGFR, relationship between urine output and day 7 eGFR, and comparison of fluid volumes received by children who did and did not experience adverse events (oxygen requirement, pulmonary oedema, systemic hypertension, and unplanned PICU admission). Potential confounders including diuretic dose, use of inotropes, type of transplant, and age were evaluated using non-parametric tests as appropriate.
All statistical analyses were performed using SPSS Statistics for Windows version 28.
Results
A total of 113 patients were identified from 5 transplant centres. Eleven were excluded due to incomplete data; thus, 102 were included in the final analysis. Forty-two (41%) were female; the median age at transplant was 12.5 (range 2–19; IQR 9) years. Fifty-six (55%) were living donor and 46 (45%) deceased donor transplants. A range of ethnicities were represented (Table 1).
Center-specific fluid management guidelines
Each participating center had specific guidelines to assist in the management of children undergoing kidney transplants. Intraoperative fluid management is at the discretion of the operating team. Central venous pressure targets vary between hospitals from 4 to 15 cmH2O. Urine output is targeted at > 2 ml/kg/h. All hospitals advocate a fluid plan that includes administration of insensible losses, in addition to the replacement of urine, stool, and other fluid losses millilitre for millilitre. There was variation between hospitals on the calculation for insensible losses with 400 ml/m2/day, 20 ml/m2/h, and 300 ml/m2/day.
Fluid administered perioperatively
The median total volume of fluid administered, including intraoperatively and the first 72 h posttransplant, was 377 ml/kg (range 122–1184 ml/kg; IQR 149 ml/kg).
Fluid administered intraoperatively
The median volume of fluid administered intraoperatively was 66 ml/kg (IQR 52 ml/kg) (Fig. 1).
Fluid administered in the 3 days postoperatively
The daily fluid volume administered decreased from a median of 148 ml/kg (range 23–786 ml/kg) on day 1 to a median of 70 ml/kg (range 9–194 ml/kg) on day 3 postoperatively (Fig. 2).
Comparison of fluid volumes administered to smaller (< 20 kg) vs. larger recipients
Children weighing less than 20 kg received a median of 507 ml/kg total fluid volume, compared with 356 ml/kg for children ≥ 20 kg (p < 0.001).
Intraoperative fluid volumes were significantly greater for children < 20 kg (median 104 ml/kg vs. 56 ml/kg; p < 0.001).
Postoperative fluid administered (excluding intraoperative fluid) was also greater for children under 20 kg (410 ml/kg vs. 294 ml/kg; p < 0.001).
Use of diuretics and inotropic support
Sixty-six (65%) children received furosemide intraoperatively with a median dose administered of 1.5 mg/kg (IQR 1.1). Twenty-nine children (28%) were given mannitol at a median dose of 500 mg/kg (IQR 156). Postoperatively furosemide was given to 39 (38%) children with a median dose of 1 (IQR 1.1) mg/kg.
Inotropes were administered intraoperatively in 68 (67%) children and postoperatively in 39 (38%) children.
Kidney transplant function
All grafts survived.
The median estimated glomerular filtration rate at day 7 was 84 (IQR 57.6) ml/min/1.73 m2. The median total urine output over the whole operative period was 255 ml/kg (IQR 156 ml/kg). The total fluid volume administered exceeded urine output by 131 ml/kg (range − 86 to 496 ml/kg; p < 0.001) (Fig. 3). The total diuretic dose was positively related to urine output (p < 0.001).
Children weighing < 20 kg had median eGFR 135 ml/min/1.73 m2 (IQR 60) at day 7, compared with 70 ml/min/1.73 m2 (IQR 42.7) in those ≥ 20 kg (p < 0.001).
A negative relationship between total fluid administered and day 7 eGFR was observed (p < 0.001); children who received higher fluid volumes had lower day 7 eGFR. A similar negative relationship between the total amount of urine output and the day 7 eGFR was found (p < 0.001) (Fig. 5). Neither inotrope use nor demographic factors were significantly related to eGFR. Diuretic dose and transplant type (living vs. deceased donor) associated with eGFR (p < 0.001), however, did not explain the negative relationship between eGFR and fluid volume.
Three children experienced delayed graft function. All three received deceased donor kidneys. Two were > 20 kg with an unknown aetiology to their kidney failure; the third was < 20 kg with an underlying diagnosis of CAKUT. None received inotropic support but all three received intraoperative and postoperative diuretics. All experienced hypertension and two were admitted to PICU. All three received less than the median volume of fluid seen in the whole cohort (377 ml/kg) with the < 20 kg child receiving 283 ml/kg in total, and the > 20 kg children receiving 362 ml/kg and 238 ml/kg respectively.
Adverse events
Systemic hypertension was the predominant adverse event in 53 (52%) children. Oxygen requirement and confirmed pulmonary oedema affected 31 (30%) and 14 (14%) children respectively. Nine (9%) patients had an unexpected admission to paediatric intensive care (PICU) (Table 2). There was no significant difference in the incidence of adverse events in those < 20 kg and those ≥ 20 kg. No significant difference between fluid volumes received in children who did or did not experience these adverse events was found (Table 2, Fig. 4).
Discussion
This study shows a high degree of variability in fluid volumes administered to children during and following kidney transplant in current practice. Short-term transplant function was found to be negatively associated with both perioperative fluid volume administered, and volume of urine output (Fig. 5).
In adult kidney transplant recipients, there is a trend for liberal fluid administration with replacement of insensible losses plus supplementary fluid to achieve high central venous pressures and to replace the urine output with the overarching goal of adequate graft perfusion [10, 11]. As such in the adult population, relatively large volumes of intravenous fluid are administered perioperatively to establish and maintain perfusion to the adult donor kidney [12]. Our data suggests that in some cases copious volumes of fluid are given in this paediatric population, but there is substantial variability.
Notably, we have found a significant difference between the total amount of fluid given per kg and day 7 eGFR (p < 0.001) with a negative skew, suggesting that increasing volumes of fluid occur with decreasing day 7 eGFR. This observation was not related to diuretic dose or inotrope administration. This result contrasts findings of the 2021 retrospective analysis by Porn-Feldman et al. [13], where it was suggested that a higher intraoperative fluid volume was associated with a lower risk for delayed graft function. In the current retrospective analysis, a causal link between larger fluid volumes and lower short-term graft function cannot be inferred; this negative association may relate to behavioural practice trends—clinicians may respond to impaired transplant function by administering more intravenous fluid.
Urine output is traditionally viewed as a marker of graft function in the postoperative period following kidney transplant [14] and as such fluid may be given in increasing volume, to drive urine output. Loop diuretics may also be administered in order to increase urine output. A large proportion of the cohort (38%) received postoperative furosemide with a positive relationship between the total dose administered and urine output. These data demonstrated a significant difference between the total amount of urine output per kg and the day 7 eGFR with a negative skew. Greater volumes of urine passed are associated with lower eGFR, which suggests that urine output volume may not be a useful marker of short-term graft function as traditionally thought. There are several potential explanations for this observation. Firstly, perioperative administration of loop diuretics can cause a potent rise in urine output which temporarily reduces intravascular volume, thereby impacting graft perfusion and function. Secondly, polyuria can result from ischaemic tubular injury, which is present in varying degrees in all transplant kidneys and reduces GFR [15]. The current study was not designed to differentiate potential mechanisms, and this warrants further prospective evaluation.
All grafts survived in this study. There were three incidences of delayed graft function, all with deceased donor kidneys. Due to the small number of cases of delayed graft function, it was not possible to evaluate potential underlying factors beyond donor type. There were numerous adverse events, notably with systemic hypertension occurring in half the population. A high frequency of hypertension postoperatively has been reported previously in other studies [13, 16]. Whilst the adverse events described in this study are often associated with fluid overload [17, 18] in the current data, there was no significant difference in the median amount of fluid given to those who developed any of the adverse events and those who did not. This is an unexpected result given the increasing recognition by the critical care community that fluid overload is associated with increased morbidity and mortality [19].
The incidence of adverse events was similar in children < 20 kg and those ≥ 20 kg. A significant difference in day 7 graft function was however noted between these groups with smaller recipients having higher eGFR. This was expected due to a greater relative size mismatch of adult donor kidneys in smaller recipients.
This study was limited by its retrospective nature, non-random participant selection due to insufficient data availability in 11 (11%) participants, and restriction to five kidney transplant centres. Notwithstanding these limitations, complete data from a defined 2-year study period on 102 children clearly illustrates the current variation in practice and questions the validity of urine output as a marker of short-term graft health.
In conclusion, there is a substantial variability in the volume of fluid administered to children receiving kidney transplants in current UK practice. A negative association between fluid volume administered and short-term graft function was also observed. It also calls into question the value of using urine output as a key marker of graft function. The retrospective nature of this data precludes the determination of causal relationships, but it does emphatically highlight the need for high-quality prospective clinical trials to optimise perioperative fluid administration in children receiving kidney transplants.
Data availability
Data available upon direct request to the corresponding author.
References
Roach JP, Bock ME, Goebel J (2017) Pediatric kidney transplantation. Semin Pediatr Surg 26:233–240
Mumford L, Maxwell H, Ahmad N, Marks SD, Tizard J (2019) The impact of changing practice on improved outcomes of paediatric renal transplantation in the United Kingdom: a 25 years review. Transpl Int 32:751–761
Melih KV, Boynuegri B, Mustafa C, Nilgun A (2019) Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation. Transplant Proc 51:1096–1100
Jia H, Huang F, Zhang X, Cheng J, Chen J, Wu J (2021) Early perioperative fluid overload is associated with adverse outcomes in deceased donor kidney transplantation. Transpl Int 34:1862–1874
Nieuwenhuijs-Moeke GJ, Huijink TM, Pol RA, El Moumni M, Burgerhof JG, Struys MM, Berger SP (2019) Intraoperative fluid restriction is associated with functional delayed graft function in living donor kidney transplantation: a retrospective cohort analysis. J Clin Med 8:1587
Morkane CM, Fabes J, Banga NR, Berry PD, Kirwan CJ (2019) Perioperative management of adult cadaveric and live donor renal transplantation in the UK: a survey of national practice. Clin Kidney J 12:880–887
Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh CR (2009) Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant 24:1039–1047
Gingell-Littlejohn M, Koh H, Aitken E, Shiels PG, Geddes C, Kingsmore D, Clancy MJ (2013) Below-target postoperative arterial blood pressure but not central venous pressure is associated with delayed graft function. Transplant Proc 45:46–50
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Torricelli FC, Watanabe A, David-Neto E, Nahas WC (2014) Current management issues of immediate postoperative care in pediatric kidney transplantation. Clinics (Sao Paulo) 69(Suppl 1):39–41
Pape L, Offner G, Ehrich JH, Sasse M (2004) A single center clinical experience in intensive care management of 104 pediatric renal transplantations between 1998 and 2002. Pediatr Transplant 8:39–43
Coupe N, O’Brien M, Gibson P, de Lima J (2005) Anesthesia for pediatric renal transplantation with and without epidural analgesia–a review of 7 years experience. Paediatr Anaesth 15:220–228
Porn-Feldman H, Davidovits M, Nahum E, Weissbach A, Kaplan E, Kadmon G (2021) Fluid overload and renal function in children after living-donor renal transplantation: a single-center retrospective analysis. Pediatr Res 90:625–631
Osman Y, El-Husseini A, Kamal M, Refaie A, Sheashaa H, Sobh M (2011) Does timing of post-renal transplant diuresis affect graft survival in live-donor renal transplants? BJU Int 107:284–287
Nieuwenhuijs-Moeke GJ, Pischke SE, Berger SP, Sanders JSF, Pol RA, Struys M, Ploeg RJ, Leuvenink HGD (2020) Ischemia and reperfusion injury in kidney transplantation: relevant mechanisms in injury and repair. J Clin Med 9:253
Baluarte HJ, Gruskin AB, Ingelfinger JR, Stablein D, Tejani A (1994) Analysis of hypertension in children post renal transplantation–a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Pediatr Nephrol 8:570–573
De Gasperi A, Narcisi S, Mazza E, Bettinelli L, Pavani M, Perrone L, Grugni C, Corti A (2006) Perioperative fluid management in kidney transplantation: is volume overload still mandatory for graft function? Transplant Proc 38:807–809
Chan W, Bosch JA, Jones D, McTernan PG, Inston N, Moore S, Kaur O, Phillips AC, Borrows R (2014) Hypervolemia and blood pressure in prevalent kidney transplant recipients. Transplantation 98:320–327
Alobaidi R, Morgan C, Basu RK, Stenson E, Featherstone R, Majumdar SR, Bagshaw SM (2018) Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr 172:257–268
Acknowledgements
All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author information
Authors and Affiliations
Contributions
NW and WH designed the study in collaboration with the other authors. All collected data. NW and WH drafted the manuscript, and all authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Ethics approval
Local ethics committee approval with consent waived due to retrospective, anonymised design.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Graphical Abstract
(PDF 190 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wyatt, N., Norman, K., Ryan, K. et al. Perioperative fluid management and associated complications in children receiving kidney transplants in the UK. Pediatr Nephrol 38, 1299–1307 (2023). https://doi.org/10.1007/s00467-022-05690-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-022-05690-3