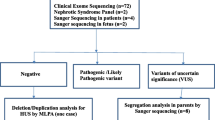
Abstract
Background
Inherited tubulopathies are a heterogeneous group of genetic disorders making whole-exome sequencing (WES) the preferred diagnostic methodology.
Methods
This was a multicenter descriptive study wherein children (< 18 years) with clinically suspected tubular disorders were recruited for molecular testing through WES. Multiplex ligation-dependent probe amplification (MLPA) and Sanger sequencing were done when required. Variants were classified as per American College of Medical Genetics 2015 guidelines and pathogenic (P)/likely pathogenic (LP) variants were considered causative.
Results
There were 77 index cases (male =73%). Median age at diagnosis was 48 months (IQR 18.5 to 108 months). At recruitment, the number of children in each clinical group was as follows: distal renal tubular acidosis (dRTA) = 25; Bartter syndrome = 18; isolated hypophosphatemic rickets (HP) = 6; proximal tubular dysfunction (pTD) = 12; nephrogenic diabetes insipidus (NDI) = 6; kidney stone/nephrocalcinosis (NC) = 6; others = 4. We detected 55 (24 novel) P/LP variants, providing genetic diagnoses in 54 children (70%). The diagnostic yield of WES was highest for NDI (100%), followed by HP (83%; all X-linked HP), Bartter syndrome (78%), pTD (75%), dRTA (64%), and NC (33%). Molecular testing had a definite impact on clinical management in 24 (31%) children. This included revising clinical diagnosis among 14 children (26% of those with a confirmed genetic diagnosis and 18% of the overall cohort), detection of previously unrecognized co-morbidities among 8 children (sensorineural deafness n = 5, hemolytic anemia n = 2, and dental changes n = 1) and facilitating specific medical treatment for 7 children (primary hyperoxaluria n = 1, cystinosis n = 4, tyrosinemia n = 2).
Conclusion
WES is a powerful tool in the diagnosis and management of children with inherited tubulopathies in the Indian population.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information

Similar content being viewed by others
Data availability
The authors follow the policy of type 2 research data (data sharing and evidence of data sharing).
References
Hoenig MP, Zeidel ML (2014) Homeostasis, the milieu intérieur, and the wisdom of the nephron. Clin J Am Soc Nephrol 9:1272–1281. https://doi.org/10.2215/CJN.08860813
Downie ML, Lopez Garcia SC, Kleta R, Bockenhauer D (2021) Inherited tubulopathies of the kidney: insights from genetics. Clin J Am Soc Nephrol 16:620–630. https://doi.org/10.2215/CJN.14481119
Hay E, Cullup T, Barnicoat A (2021) A practical approach to the genomics of kidney disorders. Pediatr Nephrol. https://doi.org/10.1007/s00467-021-04995-z
Iancu D, Ashton E (2020) Inherited renal tubulopathies-challenges and controversies. Genes (Basel) 11:277. https://doi.org/10.3390/genes11030277
Gulati A, Somlo S (2018) Whole exome sequencing: a state-of-the-art approach for defining (and exploring!) genetic landscapes in pediatric nephrology. Pediatr Nephrol 33:745–761. https://doi.org/10.1007/s00467-017-3698-0
Trautmann A, Lipska-Ziętkiewicz BS, Schaefer F (2018) Exploring the clinical and genetic spectrum of steroid resistant nephrotic syndrome: the PodoNet Registry. Front Pediatr 6:200. https://doi.org/10.3389/fped.2018.00200
Sampson MG, Hodgin JB, Kretzler M (2015) Defining nephrotic syndrome from an integrative genomics perspective. Pediatr Nephrol 30:51–63. https://doi.org/10.1007/s00467-014-2857-9
Trautmann A, Vivarelli M, Samuel S, Gipson D et al (2020) IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 35:1529–1561. https://doi.org/10.1007/s00467-020-04519-1
Bagga A, Khandelwal P, Mishra K, Thergaonkar R et al (2019) Hemolytic uremic syndrome in a developing country: consensus guidelines. Pediatr Nephrol 34:1465–1482. https://doi.org/10.1007/s00467-019-04233-7
Savige J, Ariani F, Mari F, Bruttini M et al (2019) Expert consensus guidelines for the genetic diagnosis of Alport syndrome. Pediatr Nephrol 34:1175–1189. https://doi.org/10.1007/s00467-018-3985-4
Ashton EJ, Legrand A, Benoit V, Roncelin I et al (2018) Simultaneous sequencing of 37 genes identified causative mutations in the majority of children with renal tubulopathies. Kidney Int 93:961–967. https://doi.org/10.1016/j.kint.2017.10.016
Lopez-Garcia SC, Emma F, Walsh SB, Fila M et al (2019) Treatment and long-term outcome in primary distal renal tubular acidosis. Nephrol Dial Transplant 34:981–991. https://doi.org/10.1093/ndt/gfy409
Walsh PR, Tse Y, Ashton E, Lancu D et al (2018) Clinical and diagnostic features of Bartter and Gitelman syndromes. Pediatr Clin North Am 66:121–134. https://doi.org/10.1016/j.pcl.2018.08.010
Palazzo V, Provenzano A, Becherucci F, Sansavini G et al (2017) The genetic and clinical spectrum of a large cohort of patients with distal renal tubular acidosis. Kidney Int 91:1243–1255. https://doi.org/10.1016/j.kint.2016.12.017
Gómez J, Gil-Peña H, Santos F, Coto E et al (2018) Primary distal renal tubular acidosis: novel findings in patients studied by next-generation sequencing. Pediatr Res 83:190. https://doi.org/10.1038/pr.2017.242
Nagara M, Voskarides K, Nouira S, Halim NB et al (2014) Molecular investigation of distal renal tubular acidosis in Tunisia, evidence for founder mutations. Genet Test Mol Biomarkers 18:741–748. https://doi.org/10.1089/gtmb.2014.0175
Gu J, Wang C, Zhang H, Yue H et al (2018) Targeted resequencing of phosphorus metabolism-related genes in 86 patients with hypophosphatemic rickets/osteomalacia. Int J Mol Med 42:1603–1614. https://doi.org/10.3892/ijmm.2018.3730
Sharma PK, Saikia B, Sharma R, Ankur K et al (2014) Genetic analysis in Bartter syndrome from India. Indian J Pediatr 81:1095–1098. https://doi.org/10.1007/s12098-014-1379-6
Sethi SK, Bagga A, Gulati A, Hari P et al (2008) Mutations in OCRL1 gene in Indian children with Lowe syndrome. Clin Exp Nephrol 12:358–362. https://doi.org/10.1007/s10157-008-0059-0
Amita M, Srivastava P, Mandal K, De S et al (2017) Fanconi-Bickel syndrome: another novel mutation in SLC2A2. Indian J Pediatr 84:236–237. https://doi.org/10.1007/s12098-016-2236-6
Ashton E, Bockenhauer D (2020) Diagnosis of uncertain significance: can next-generation sequencing replace the clinician? Kidney Int 97:455–457. https://doi.org/10.1016/j.kint.2019.12.012
Richards S, Aziz N, Bale S, Bick D et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–24. https://doi.org/10.1038/gim.2015.30
Das D, Sinha R, Dey S (2019) Renal tubular acidosis presenting as nephrogenic diabetes insipidus. Indian Pediatr 5:325–327
Trepiccione F, Walsh SB, Ariceta G, Boyer O et al (2021) Distal renal tubular acidosis: ERKNet/ESPN clinical practice points. Nephrol Dial Transplant 36:1585–1596. https://doi.org/10.1093/ndt/gfab171
Soares SBM, de Menezes Silva LAW, de Carvalho Mrad FC, Simões E Silva AC (2019) Distal renal tubular acidosis: genetic causes and management. World J Pediatr 15:422–431. https://doi.org/10.1007/s12519-019-00260-4
Khositseth S, Bruce LJ, Walsh SB, Bawazir WM et al (2012) Tropical distal renal tubular acidosis: clinical and epidemiological studies in 78 patients. QJM 105:861–877. https://doi.org/10.1093/qjmed/hcs139
Rungroj N, Nettuwakul C, Sawasdee N, Sangnual S et al (2018) Distal renal tubular acidosis caused by tryptophan-aspartate repeat domain 72 (WDR72) mutations. Clin Genet 94:409–418. https://doi.org/10.1111/cge.13418
Mantoo MR, Kabra M, Kabra SK (2020) Cystic fibrosis presenting as pseudo-bartter syndrome: an important diagnosis that is missed! Indian J Pediatr 87:726–732. https://doi.org/10.1007/s12098-020-03342-8
Eğrıtaş O, Dalgiç B, Wedenoja S (2011) Congenital chloride diarrhea misdiagnosed as Bartter syndrome. Turk J Gastroenterol 22:321–323
Ben-David Y, Halevy R, Sakran W, Zehavi Y et al (2019) The utility of next generation sequencing in the correct diagnosis of congenital hypochloremic hypokalemic metabolic alkalosis. Eur J Med Genet 62:103728. https://doi.org/10.1016/j.ejmg.2019.103728
Raut S, Khandelwal P, Sinha A, Thakur R et al (2020) Infantile nephropathic cystinosis: clinical features and outcome. Asian J Pediatr Nephrol 3:15. https://doi.org/10.4103/AJPN.AJPN_10_20
Bockenhauer D, van’t Hoff W, Dattani M, Lehnhardt A et al (2010) Secondary nephrogenic diabetes insipidus as a complication of inherited renal diseases. Nephron Physiol 116:23–29. https://doi.org/10.1159/000320117
Kalyanasundaram SKK (2016) Autosomal recessive distal renal tubular acidosis with secondary nephrogenic diabetes insipidus in newborn a case report. Univ J Med Med Spec http://ejournal-tnmgrmu.ac.in/index.php/medicine/article/view/1438. Accessed 18 September 2021
Nagayama Y, Shigeno M, Nakagawa Y, Suganuma A et al (1994) Acquired nephrogenic diabetes insipidus secondary to distal renal tubular acidosis and nephrocalcinosis associated with Sjögren’s syndrome. J Endocrinol Invest 17:659–663. https://doi.org/10.1007/BF03349682
Khandelwal P, Sabanadesan J, Sinha A, Hari P et al (2020) Isolated nephrocalcinosis due to compound heterozygous mutations in renal outer medullary potassium channel. CEN Case Rep 9:232–236. https://doi.org/10.1007/s13730-020-00464-y
Amar A, Majmundar AJ, Ullah I, Afzal A et al (2019) Gene panel sequencing identifies a likely monogenic cause in 7% of 235 Pakistani families with nephrolithiasis. Hum Genet 138:211–219. https://doi.org/10.1007/s00439-019-01978-x
Daga A, Majmundar AJ, Braun DA, Gee HY et al (2018) Whole exome sequencing frequently detects a monogenic cause in early onset nephrolithiasis and nephrocalcinosis. Kidney Int 93:204–213. https://doi.org/10.1016/j.kint.2017.06.025
Adalat S, Woolf AS, Johnstone KA, Wirsing A et al (2009) HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 20:1123–1131. https://doi.org/10.1681/ASN.2008060633
Adalat S, Hayes WN, Bryant WA, Booth J et al (2019) HNF1B mutations are associated with a Gitelman-like tubulopathy that develops during childhood. Kidney Int Rep 4:1304–1311. https://doi.org/10.1016/j.ekir.2019.05.019
Carboni I, Andreucci E, Caruso MR, Ciccone R et al (2009) Medullary sponge kidney associated with primary distal renal tubular acidosis and mutations of the H + -ATPase genes. Nephrol Dial Transplant 24:2734–2738. https://doi.org/10.1093/ndt/gfp160
Wagner CA, Devuyst O, Belge H, Bourgeois S et al (2011) The rhesus protein RhCG: a new perspective in ammonium transport and distal urinary acidification. Kidney Int 79:154–161. https://doi.org/10.1038/ki.2010.386
Acknowledgements
We acknowledge the contributions of the patients and their families for participating in the study.
Funding
Medgenome Labs Private Limited has partially funded the genetic tests.
Author information
Authors and Affiliations
Contributions
Rajiv Sinha and Kausik Mandal contributed to conceptualization, supervision, writing, and reviewing the manuscript. All authors have contributed to data acquisition, data curation, editing, and final acceptance for submission of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Ethical approval was obtained from the Institute of Child Health Ethics Committee, Kolkata, India.
Consent to participate
Informed consent was obtained from the parents or guardians as well as from the children aged ≥ 13 years.
Consent for publication
All participants and authors consented to publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sinha, R., Pradhan, S., Banerjee, S. et al. Whole-exome sequencing and variant spectrum in children with suspected inherited renal tubular disorder: the East India Tubulopathy Gene Study. Pediatr Nephrol 37, 1811–1836 (2022). https://doi.org/10.1007/s00467-021-05388-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05388-y