Abstract
Background
Acute kidney injury (AKI) is characterized by an abrupt decline in glomerular filtration rate (GFR). We sought to identify separate early urinary metabolomic signatures at AKI onset (with-AKI) and prior to onset of functional impairment (pre-AKI).
Methods
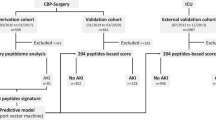
Pre-AKI (n=15), AKI (n=22), and respective controls (n=30) from two prospective PICU cohort studies provided urine samples which were analyzed by GC-MS and DI-MS mass spectrometry (193 metabolites). The cohort (n=58) was 8.7±6.4 years old and 66% male. AKI patients had longer PICU stays, higher PRISM scores, vasopressors requirement, and respiratory diagnosis and less commonly had trauma or post-operative diagnosis. Urine was collected within 2–3 days after admission and daily until day 5 or 14.
Results
The metabolite classifiers for pre-AKI samples (1.5±1.1 days prior to AKI onset) had a cross-validated area under receiver operator curve (AUC)=0.93 (95%CI 0.85–1.0); with-AKI samples had an AUC=0.94 (95%CI 0.87–1.0). A parsimonious pre-AKI classifier with 13 metabolites was similarly robust (AUC=0.96, 95%CI 0.89–1.0). Both classifiers were similar and showed modest correlation of high-ranking metabolites (tau=0.47, p<0.001).
Conclusions
This exploratory study demonstrates the potential of a urine metabolite classifier to detect AKI-risk in pediatric populations earlier than the current standard of diagnosis with the need for external validation.
Graphical abstract

A higher resolution version of the Graphical abstract is available as Supplementary information with inner reference to ESM for GA



Similar content being viewed by others
Data availability
Patient level data is not publicly available. Primary data is available upon request from the corresponding author.
Code availability
Code for analysis in R is available, upon request from the corresponding author.
References
Sutherland S, Ji J, Sheikhi F (2013) AKI in Hospitalized Children: Epidemiology and Clinical Associations in a National Cohort. Clin J Am Soc Nephrol 8:1661–1669
Benisty K, Morgan C, Hessey E, Huynh L et al (2020) Kidney and blood pressure abnormalities 6 years after acute kidney injury in critically ill children: a prospective cohort study. Pediatr Res 88:271–278. https://doi.org/10.1038/s41390-019-0737-5
Kellum JA, Lameire N, Aspelin P, Barsoum RS et al (2012) Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. https://doi.org/10.1038/kisup.2012.1
Meersch M, Schmidt C, Van Aken H, Martens S et al (2014) Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One 9:1–9. https://doi.org/10.1371/journal.pone.0093460
Martin-Lorenzo M, Gonzalez-Calero L, Ramos-Barron A, Sanchez-Niño MD et al (2017) Urine metabolomics insight into acute kidney injury point to oxidative stress disruptions in energy generation and H 2 S availability. J Mol Med 95:1399–1409. https://doi.org/10.1007/s00109-017-1594-5
Zhou F, Luo Q, Wang L, Han L (2016) Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury : a meta-analysis. Eur J Cardio-Thoracic Surg 49:746–755. https://doi.org/10.1093/ejcts/ezv199
Marx D, Metzger J, Pejchinovski M, Gil RB et al (2018) Proteomics and Metabolomics for AKI Diagnosis. Semin Nephrol 38:63–87. https://doi.org/10.1016/J.SEMNEPHROL.2017.09.007
Vanmassenhove J, Glorieux G, Lameire N, Hoste E et al (2015) Influence of severity of illness on neutrophil gelatinase-associated lipocalin performance as a marker of acute kidney injury: a prospective cohort study of patients with sepsis. BMC Nephrol 16:18. https://doi.org/10.1186/s12882-015-0003-y
Alge JL, Arthur JM (2015) Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol 10:147–155. https://doi.org/10.2215/CJN.12191213
Archdekin B, Sharma A (2019) Non-invasive differentiation of non-rejection kidney injury from acute rejection in pediatric renal transplant recipients. Pediatr Transpl 23:e13364
Schwartz GJ, Work DF (2009) Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–1843. https://doi.org/10.2215/CJN.01640309
Sharma A, Gibson IW, Mandal R, Wishart DS (2014) Urinary Metabolomics for Noninvasive Detection of Borderline and Acute T Cell – Mediated Rejection in Children After Kidney Transplantation. Am J Transpl 14:2339–2349. https://doi.org/10.1111/ajt.12837
Blydt-hansen TD, Sharma A, Gibson IW, Wishart DS et al (2017) Urinary Metabolomics for Noninvasive Detection of Antibody-Mediated Rejection in Children After Kidney Transplantation. Transplantation 101:2553–2561. https://doi.org/10.1097/TP.0000000000001662
Suhre K, Schwartz J, Sharma V, Chen Q (2016) Urine Metabolite Profiles Predictive of Human Kidney Allograft Status. J Am Soc Nephrol 27:626–636
Landsberg A, Sharma A, Gibson IW, Rush D et al (2018) Non-invasive staging of chronic kidney allograft damage using urine metabolomic profiling. Pediatr Transplant 22:e13226. https://doi.org/10.1111/petr.13226
Bojko B, Marcin W, Pawliszyn J (2014) Metabolic pro fi ling of plasma from cardiac surgical patients concurrently administered with tranexamic acid : DI-SPME – LC – MS analysis. J Pharm Anal 4:6–13. https://doi.org/10.1016/j.jpha.2013.03.002
Simonato M, Fochi I, Vedovelli L, Giambelluca S et al (2019) Urinary metabolomics reveals kynurenine pathway perturbation in newborns with transposition of great arteries after surgical repair. Metabolomics 15:145. https://doi.org/10.1007/s11306-019-1605-3
Lee C, Hsieh Y, Chen S, Fu S et al (2018) Bretschneider solution- induced alterations in the urine metabolome in cardiac surgery patients. Sci Rep 8:17774. https://doi.org/10.1038/s41598-018-35631-w
Lagos-Arevalo P, Palijan A, Vertullo L, Devarajan P et al (2015) Cystatin C in acute kidney injury diagnosis: early biomarker or alternative to serum creatinine? Pediatr Nephrol 30:665–676
Palermo J, Dart AB, De Mello A, Devarajan P et al (2017) Biomarkers for Early Acute Kidney Injury Diagnosis and Severity Prediction: A Pilot Multicenter Canadian Study of Children Admitted to the ICU. Pediatr Crit Care Med 18:e235–e244. https://doi.org/10.1097/PCC.0000000000001183
Eric Thomas C, Sexton W, Benson K, Sutphen R et al (2010) Urine collection and processing for protein biomarker discovery and quantification. Cancer Epidemiol Biomarkers Prev 19:953–959. https://doi.org/10.1158/1055-9965.EPI-10-0069
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK et al (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71:1028–1035. https://doi.org/10.1038/sj.ki.5002231
Schwartz GJ, Muñoz A, Schneider MF, Mak RH et al (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. https://doi.org/10.1681/ASN.2008030287
Rink N, Zappitelli M (2015) Estimation of glomerular filtration rate with and without height: effect of age and renal function level. Pediatr Nephrol 30:1327–1336
Zheng J, Mandal R, Wishart DS (2018) A sensitive, high-throughput LC-MS / MS method for measuring catecholamines in low volume serum. Anal Chim Acta 1037:159–167. https://doi.org/10.1016/j.aca.2018.01.021
Bouatra S, Aziat F, Mandal R, Guo AC et al (2013) The Human Urine Metabolome. PLoS One 8:e73076. https://doi.org/10.1371/journal.pone.0073076
Vogl FC, Mehrl S, Heizinger L, Schlecht I et al (2016) Evaluation of dilution and normalization strategies to correct for urinary output in HPLC-HRTOFMS metabolomics. Anal Bioanal Chem 408:8483–8493. https://doi.org/10.1007/s00216-016-9974-1
Lê Cao KA, Boitard S, Besse P (2011) Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinformatics 12:253. https://doi.org/10.1186/1471-2105-12-253
Xia J, Mandal R, Sinelnikov IV, Broadhurst D et al (2012) MetaboAnalyst 2.0—a comprehensive server for metabolomic data analysis. Nucleic Acids Res 40:127–133. https://doi.org/10.1093/nar/gks374
Melvik B-H, Wehrens R (2007) The pls Package: Principal Component and Partial Least Squares Regression in R. J Stat Softw 18:1–23
De Abreu KLS, Da Silva GB, Muniz TD, Barreto AGC et al (2013) Acute kidney injury in critically ill patients with lung disease: Kidney-lung crosstalk. Rev Bras Ter Intensiva 25:130–136. https://doi.org/10.5935/0103-507X.20130024
(2012) Section 3: Prevention and Treatment of AKI. Kidney Int Suppl 2:37–68. https://doi.org/10.1038/kisup.2011.33
Paladino JD, Hotchkiss JR, Rabb H (2009) Acute kidney injury and lung dysfunction: A paradigm for remote organ effects of kidney disease? Microvasc Res 77:8–12. https://doi.org/10.1016/j.mvr.2008.09.001
Kashani K, Al-Khafaji A, Ardiles T, Artigas A et al (2013) Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17:R25. https://doi.org/10.1186/cc12503
Beger RD, Holland RD, Sun J, Schnackenberg LK et al (2008) Metabonomics of acute kidney injury in children after cardiac surgery. Pediatr Nephrol 23:977–984. https://doi.org/10.1007/s00467-008-0756-7
Muhle-goll C, Eisenmann P, Luy B, Kölker S et al (2020) Urinary NMR profiling in pediatric acute kidney injury—a pilot study. Int J Mol Sci 21:1187. https://doi.org/10.3390/ijms21041187
Mercier K, McRitchie S, Pathmasiri W, Novokhatny A (2017) Preterm Neonatal Urinary Renal Developmental and Acute Kidney Injury Metabolomic Profiling: An Exploratory Study. Pediatr Nephrol 32:151–161. https://doi.org/10.1016/j.physbeh.2017.03.040
Chesney RW, Han X, Patters AB (2010) Taurine and the renal system. J Biomed Sci 17 Suppl 1(Suppl 1):S4. https://doi.org/10.1186/1423-0127-17-S1-S4
Trachtman H, Futterweit S, Prenner J, Hanon S (1994) Antioxidants reverse the antiproliferative effect of high glucose and advanced glycosylation end products in cultured rat mesangial cells. Biochem Biophys Res Commun 199:346–352
Jouret F, Leenders J, Poma L, Defraigne JO et al (2016) Nuclear magnetic resonance metabolomic profiling of mouse kidney, urine and serum following renal ischemia/reperfusion injury. PLoS One 11:e0163021. https://doi.org/10.1371/journal.pone.0163021
Qu X, Gao H, Sun J, Tao L et al (2020) Identification of key metabolites during cisplatin-induced acute kidney injury using an HPLC-TOF/MS-based non-targeted urine and kidney metabolomics approach in rats. Toxicology 431:152366. https://doi.org/10.1016/j.tox.2020.152366
Cooke D, Ouattara A, Ables GP (2018) Dietary methionine restriction modulates renal response and attenuates kidney injury in mice. FASEB J 32:693–702. https://doi.org/10.1096/fj.201700419R
Kim YS, Jung MH, Choi MY, Kim YH et al (2009) Glutamine attenuates tubular cell apoptosis in acute kidney injury via inhibition of the c-Jun N-terminal kinase phosphorylation of 14–3-3. Crit Care Med 37:2033–2044. https://doi.org/10.1097/CCM.0b013e3181a005ba
Dalili N, Chashmniam S, Khoormizi SMH, Salehi L et al (2020) Urine and serum NMR-based metabolomics in pre-procedural prediction of contrast-induced nephropathy. Intern Emerg Med 15:95–103. https://doi.org/10.1007/s11739-019-02128-x
Vera-Aviles M, Vantana E, Kardinasari E, Koh NL et al (2018) Protective role of histidine supplementation against oxidative stress damage in the management of anemia of chronic kidney disease. Pharmaceuticals 11:111. https://doi.org/10.3390/ph11040111
Wang S, Xiao C, Liu C, Li J et al (2020) Identification of Biomarkers of Sepsis-Associated Acute Kidney Injury in Pediatric Patients Based on UPLC-QTOF/MS. Inflammation 43:629–640. https://doi.org/10.1007/s10753-019-01144-5
Zhao YY (2013) Metabolomics in chronic kidney disease. Clin Chim Acta 422:59–69. https://doi.org/10.1016/j.cca.2013.03.033
Späth MR, Bartram MP, Palacio-Escat N, Hoyer KJR et al (2019) The proteome microenvironment determines the protective effect of preconditioning in cisplatin-induced acute kidney injury. Kidney Int 95:333–349. https://doi.org/10.1016/j.kint.2018.08.037
Aregger F, Uehlinger DE, Fusch G, Bahonjic A et al (2018) Increased urinary excretion of kynurenic acid is associated with non-recovery from acute kidney injury in critically ill patients. BMC Nephrol 19:44. https://doi.org/10.1186/s12882-018-0841-5
Hanna MH, Segar JL, Teesch LM, Kasper DC et al (2013) Urinary metabolomic markers of aminoglycoside nephrotoxicity in newborn rats. Pediatr Res 73:585–591. https://doi.org/10.1038/pr.2013.34.Urinary
Acknowledgements
We are grateful to the patients and parents who agreed to participate in the two primary studies on acute kidney injury.
Funding
This work was funded by an establishment grant by the BC Children’s Hospital Foundation. The primary research was funded by McGill University Health Centre Research Institute, the Kidney Research Scientist Core Education, National Training Program and the Fonds de Recherches en Santé du Quebec, a research salary award from the Fonds de Recherches du Quebec-Sante, and grants from the National Institutes of Health (NIH, P50DK096418).
Author information
Authors and Affiliations
Contributions
The following authors were involved in conceiving the research concept and approving the final manuscript: TBH, MZ, and AS. Analysis and results reporting was completed by AS, TBH, VC, and AF. The manuscript was drafted by AF.
Corresponding author
Ethics declarations
Ethics approval
Research ethics board approval was obtained from the University of British Columbia. In the primary studies, research ethics board approval was obtained to perform research activities at all participating institutions.
Consent to participate
Informed consent (and child assent when appropriate) in the original studies was obtained and included consent to utilize frozen biologic specimens for future analyses.
Consent for publication
Patients consented regarding publishing their data as per primary studies.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Franiek, A., Sharma, A., Cockovski, V. et al. Urinary metabolomics to develop predictors for pediatric acute kidney injury. Pediatr Nephrol 37, 2079–2090 (2022). https://doi.org/10.1007/s00467-021-05380-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05380-6