Abstract
Background
Sodium depletion results in impaired somatic growth. The sodium requirements of extremely preterm (periviable) infants early in life are not known. We therefore investigated sodium homeostasis in this population over the first 10 weeks following birth.
Methods
This was a longitudinal, observational study of sodium intake and urine sodium excretion in a convenience cohort of 23 infants born at 22 0/7–23 6/7-week gestation.
Results
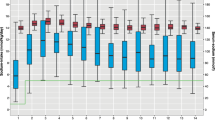
Sodium intake ranged from 5.2 ± 0.4 to a maximum of 7.9 ± 0.5 mEq/kg/day at 2 and 8 weeks of postnatal age, respectively, while urinary sodium loss was 7.7 ± 1.0 mEq/kg/day and 6.9 ± 0.7 mEq/kg/day at these time points. Sodium balance (sodium intake – urine sodium output) was first positive at 6 weeks of age, though a positive sodium balance exceeding 1.4 mEq/kg/day (i.e., a balance associated with weight gain of 30 g/day) was not observed until 10 weeks.
Conclusions
Infants born at 22–23-week gestational age have a prolonged period of high urinary losses of sodium and negative sodium balance. Sodium intakes greater than those currently recommended by the American Academy of Pediatrics are needed to achieve a significant positive sodium balance in this population.
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information.


Similar content being viewed by others
Change history
06 November 2021
ESM was extended to include the article's Graphical abstract
References
Segar JL (2017) Renal adaptive changes and sodium handling in the fetal-to-newborn transition. Semin Fetal Neonatal Med 22:76–82. https://doi.org/10.1016/j.siny.2016.11.002
Quigley R (2018) Renal aspects of sodium metabolism in the fetus and neonate. In: Oh W, Baum M (eds) Neonatology questions and controversies: nephrology and fluid/electrolyte physiology. Elsevier, Philadelphia, pp 47–64
Bueva A, Guignard JP (1994) Renal function in preterm neonates. Pediatr Res 36:572–577. https://doi.org/10.1203/00006450-199411000-00005
Siegel SR, Oh W (1976) Renal function as a marker of human fetal maturation. Acta Paediatr Scand 65:481–485. https://doi.org/10.1111/j.1651-2227.1976.tb04917.x
Späth C, Sjöström ES, Ahlsson F, Ågren J, Domellöf M (2017) Sodium supply influences plasma sodium concentration and the risks of hyper- and hyponatremia in extremely preterm infants. Pediatr Res 81:455–460. https://doi.org/10.1038/pr.2016.264
Fine BP, Ty A, Lestrange N, Levine OR (1987) Sodium deprivation growth failure in the rat: alterations in tissue composition and fluid spaces. J Nutr 117:1623–1628. https://doi.org/10.1093/jn/117.9.1623
Wassner SJ (1989) Altered growth and protein turnover in rats fed sodium-deficient diets. Pediatr Res 26:608–613. https://doi.org/10.1203/00006450-198912000-00019
Wassner SJ (1991) The effect of sodium repletion on growth and protein turnover in sodium-depleted rats. Pediatr Nephrol 5:501–504. https://doi.org/10.1007/BF01453690
Chou JH, Roumiantsev S, Singh R (2020) PediTools Electronic Growth Chart Calculators: applications in clinical care, research, and quality improvement. J Med Internet Res 22:e16204. https://doi.org/10.2196/16204
Al-Dahhan J, Haycock GB, Chantler C, Stimmler L (1983) Sodium homeostasis in term and preterm neonates. I. Renal aspects. Arch Dis Child 58:335–342. https://doi.org/10.1136/adc.58.5.335
Gallini F, Maggio L, Romagnoli C, Marrocco G, Tortorolo G (2000) Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr Nephrol 15:119–124. https://doi.org/10.1007/s004670000356
Gubhaju L, Sutherland MR, Horne RS, Medhurst A, Kent AL, Ramsden A et al (2014) Assessment of renal functional maturation and injury in preterm neonates during the first month of life. Am J Physiol Ren Physiol 307:F149–F158. https://doi.org/10.1152/ajprenal.00439.2013
Delgado MM, Rohatgi R, Khan S, Holzman IR, Satlin LM (2003) Sodium and potassium clearances by the maturing kidney: clinical-molecular correlates. Pediatr Nephrol 18:759–767. https://doi.org/10.1007/s00467-003-1178-1
Segar DE, Segar EK, Harshman LA, Dagle JM, Carlson SJ, Segar JL (2018) Physiological approach to sodium supplementation in preterm infants. Am J Perinatol 35:994–1000. https://doi.org/10.1055/s-0038-1632366
Isemann B, Mueller EW, Narendran V, Akinbi H (2016) Impact of early sodium supplementation on hyponatremia and growth in premature infants: a randomized controlled trial. J Parenter Enter Nutr 40:342–349. https://doi.org/10.1177/0148607114558303
Acknowledgements
The authors wish to acknowledge Mendi Schmelzel, MSN-RN, RNC-NIC, and Karen Johnson, RN BSN, for their assistance in gathering data and the families of our NICU patients for participating in this project.
Funding
No funding source or sponsorship supported original collection of data; however, JL Grobe is now supported by grants from the NIH (HL134850, HL084207), the American Heart Association (18EIA33890055), the MCW Clinical & Translational Science Institute “Obesity” Ensemble (UL1TR001436), and the Advancing a Healthier Wisconsin Endowment to MCW.
Author information
Authors and Affiliations
Contributions
JS is responsible for conceptualizing the study, methodology, data curation, and analysis and wrote the initial draft of the manuscript. CG participated in data analysis and interpretation and in revising the draft manuscript. JG contributed to study methodology, data curation, and analysis and in revising the draft manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approval for this longitudinal cohort study was approved by the University of Iowa Human Subjects Institutional Review Board.
Consent to participate
Informed consent was obtained for parents of subjects according to Institutional Review Board policies.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
J Segar and J Grobe were affiliated with the University of Iowa at the time the data were collected.
Supplementary Information
Graphical abstract
(PPTX 70.3 kb)
Rights and permissions
About this article
Cite this article
Segar, J.L., Grobe, C.C. & Grobe, J.L. Maturational changes in sodium metabolism in periviable infants. Pediatr Nephrol 36, 3693–3698 (2021). https://doi.org/10.1007/s00467-021-05119-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-021-05119-3