Abstract
Background
Rejection is responsible for just under 50% of graft loss in the pediatric kidney transplant population. Early identification and treatment of allograft injury, specifically modifiable pathologies such as subclinical rejection (SCR), calcineurin inhibitor toxicity, and BK virus nephropathy, may improve allograft survival. Protocol surveillance biopsy (SB) currently offers the earliest opportunity for targeted interventions.
Methods
This is a single-center retrospective review of 215 kidney SBs obtained from 2008 to 2016 in 97 pediatric kidney transplant recipients. SBs were obtained at 6, 12, and 24 months post-transplantation. Frequency of abnormal histologic findings, estimated glomerular filtration rate at time of SB, and SB-related complications were recorded. Data were analyzed to investigate possible time trends and the presence of demographic or clinical associations with abnormal histologic findings.
Results
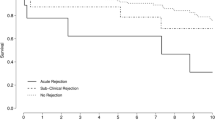
Potentially modifiable histologic findings were seen in 38.1% of all SBs. SCR was found with increasing frequency across all time points with an estimated 49% increase in the odds of a SCR finding per additional 6 months post-transplantation (aOR 1.49, 95% CI 1.06–2.09, p = 0.022). Among follow-up biopsies in patients who underwent treatment for SCR, 50% had no SCR and 18.8% showed histologic improvement. The complication rate associated with SB was 1.9% (4/215 SBs) and consisted of only minor complications.
Conclusions
SBs are safe and offer the opportunity to identify and treat modifiable histologic changes in the pediatric kidney transplant population. The performance of SBs for up to 2 years after transplantation can have meaningful clinical impact.


Similar content being viewed by others
References
National Data - OPTN. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. Accessed 24 Oct 2019
Karcz M, Kusztal M, Boratyńska M, Klinger M (2018) Very long-term survival of the transplanted kidney-characteristics of recipients. Transplant Proc 50:1730–1732. https://doi.org/10.1016/j.transproceed.2018.02.114
Sert I, Yavascan Ö, Tugmen C, Orhan DK, Kilinc S, Dogan SM, Bal A, Kebabci E, Alparslan C, Karaca C, Aksu N (2013) A retrospective analysis of long-term graft survival in 61 pediatric renal transplant recipients: a single-center experience. Ann Transplant 18:497–504. https://doi.org/10.12659/AOT.889117
Naderi G, Latif A, Karimi S, Tabassomi F, Esfahani ST (2017) The long-term outcome of pediatric kidney transplantation in Iran: results of a 25-year single-center cohort study. Int J Organ Transplant Med 8:85–96
Hebert SA, Swinford RD, Hall DR, Au JK, Bynon JS (2017) Special considerations in pediatric kidney transplantation. Adv Chronic Kidney Dis 24:398–404. https://doi.org/10.1053/j.ackd.2017.09.009
Graves RC, Fine RN (2016) Kidney retransplantation in children following rejection and recurrent disease. Pediatr Nephrol 31:2235–2247
Cihan Y, Kanzelmeyer N, Drube J, Kreuzer M, Lerch C, Hennies I, Froede K, Verboom M, Ahlenstiel-Grunow T, Pape L (2017) Rabbit anti-human thymocyte immunoglobulin for the rescue treatment of chronic antibody-mediated rejection after pediatric kidney transplantation. Pediatr Nephrol 32:2133–2142. https://doi.org/10.1007/s00467-017-3725-1
Moreso F, Ibernon M, Gomà M, Carrera M, Fulladosa X, Hueso M, Gil-Vernet S, Cruzado JM, Torras J, Grinyó SD (2006) Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant 6:747–752. https://doi.org/10.1111/j.1600-6143.2005.01230.x
Kanzelmeyer NK, Ahlenstiel T, Drube J, Froede K, Kreuzer M, Broecker V, Ehrich JHH, Melk A, Pape L (2010) Protocol biopsy-driven interventions after pediatric renal transplantation. Pediatr Transplant 14:1012–1018. https://doi.org/10.1111/j.1399-3046.2010.01399.x
Seikku P, Krogerus L, Jalanko H, Holmberg C (2005) Better renal function with enhanced immunosuppression and protocol biopsies after kidney transplantation in children. Pediatr Transplant 9:754–762
Gordillo R, Munshi R, Monroe EJ, Shivaram GM, Smith JM (2019) Benefits and risks of protocol biopsies in pediatric renal transplantation. Pediatr Nephrol 34:593–598. https://doi.org/10.1007/s00467-018-3959-6
Rose EM, Kennedy SE, Mackie FE (2016) Surveillance biopsies after paediatric kidney transplantation: a review. Pediatr Transplant 20:748–755. https://doi.org/10.1111/petr.12733
Zotta F, Guzzo I, Morolli F, Diomedi-Camassei F, Strologo LD (2018) Protocol biopsies in pediatric renal transplantation: a precious tool for clinical management. Pediatr Nephrol 33:2167–2175. https://doi.org/10.1007/s00467-018-4007-2
Rush D, Arlen D, Boucher A, Busque S, Cockfield SM, Girardin C, Knoll G, Lachance JG, Landsberg D, Shapiro J, Shoker A, Yilmaz Y (2007) Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: a randomized study. Am J Transplant 7:2538–2545. https://doi.org/10.1111/j.1600-6143.2007.01979.x
Mehta R, Sood P, Hariharan S (2016) Subclinical rejection in renal transplantation: reappraised. Transplantation 100:1610–1618. https://doi.org/10.1097/TP.0000000000001163
Henderson LK, Nankivell BJ, Chapman JR (2011) Surveillance protocol kidney transplant biopsies: their evolving role in clinical practice. Am J Transplant 11:1570–1575. https://doi.org/10.1111/j.1600-6143.2011.03677.x
Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MCR, David DSR, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB III, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M (2014) Banff 2013 Meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14:272–283. https://doi.org/10.1111/ajt.12590
Kambham N, Nagarajan S, Shah S, Li L, Salvatierra O, Sarwal MM (2007) A novel, semiquantitative, clinically correlated calcineurin inhibitor toxicity score for renal allograft biopsies. Clin J Am Soc Nephrol 2:135–142. https://doi.org/10.2215/CJN.01320406
Nickeleit V, Singh HK, Randhawa P, Drachenberg CB, Bhatnagar R, Bracamonte E, Chang A, Chon WJ, Dadhania D, Davis VG, Hopfer H, Mihatsch MJ, Papadimitriou JC, Schaub S, Stokes MB, Tungekar MF, Seshan SV (2018) The Banff Working Group Classification of Definitive Polyomavirus Nephropathy: morphologic definitions and clinical correlations. J Am Soc Nephrol 29:680–693. https://doi.org/10.1681/ASN.2017050477
Mian AN, Schwartz GJ (2017) Measurement and estimation of glomerular filtration rate in children. Adv Chronic Kidney Dis 24:348–356. https://doi.org/10.1053/j.ackd.2017.09.011
de Souza V, Cochat P, Rabilloud M, Selistre L, Wagner M, Hadj-Aissa A, Dolomanova O, Ranchin B, Iwaz J, Dubourg L (2015) Accuracy of different equations in estimating GFR in pediatric kidney transplant recipients. Clin J Am Soc Nephrol 10:463–470. https://doi.org/10.2215/CJN.06300614
Selistre L, De Souza V, Cochat P, Antonello ICF, Hadj-Aissa A, Ranchin B, Dolomanova O, Varennes A, Beyerle F, Bachetta J, Dubourg L (2012) GFR estimation in adolescents and young adults. J Am Soc Nephrol 23:989–996. https://doi.org/10.1681/ASN.2011070705
Selistre L, Rabilloud M, Cochat P, de Souza V, Iwaz J, Lemoine S, Beyerle F, Poli-de-Figueiredo CE, Dubourg L (2016) Comparison of the Schwartz and CKD-EPI equations for estimating glomerular filtration rate in children, adolescents, and adults: a retrospective cross-sectional study. PLoS Med 13:e1001979. https://doi.org/10.1371/journal.pmed.1001979
Alkandari O, Hebert D, Langlois V, Robinson LA, Parekh RS (2017) Validation of serum creatinine-based formulae in pediatric renal transplant recipients. Pediatr Res 82:1000–1006. https://doi.org/10.1038/pr.2017.209
Birk PE (2012) Surveillance biopsies in children post-kidney transplant. Pediatr Nephrol 27:753–760. https://doi.org/10.1007/s00467-011-1969-8
Dharnidharka VR, Vyas N, Gaut JP, Walther L, Hmiel SP (2018) The utility of surveillance biopsies in pediatric kidney transplantation. Pediatr Nephrol 33:889–895. https://doi.org/10.1007/s00467-017-3864-4
Birk PE, Blydt-Hansen TD, Dart AB, Kaita LM, Proulx C, Taylor G (2007) Low incidence of adverse events in outpatient pediatric renal allograft biopsies. Pediatr Transplant 11:196–200
Nazario M, Nicoara O, Becton L, Self S, Hill J, Mack E, Evans M, Twombley K (2018) Safety and utility of surveillance biopsies in pediatric kidney transplant patients. Pediatr Transplant 22:e13178. https://doi.org/10.1111/petr.13178
Rush DN, Henry SF, Jeffery JR, Schroeder TJ, Gough J (1994) Histological findings in early routine biopsies of stable renal allograft recipients. Transplantation 57:208–211
Rush D (2006) Protocol transplant biopsies: an underutilized tool in kidney transplantation. Clin J Am Soc Nephrol 1:138–143. https://doi.org/10.2215/CJN.00390705
Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA (2011) Association between age and graft failure rates in young kidney transplant recipients. Transplantation 92:1237. https://doi.org/10.1097/TP.0b013e31823411d7
Tanabe T (2014) The value of long-term protocol biopsies after kidney transplantation. Nephrology (Carlton) 19(Suppl 3):2–5. https://doi.org/10.1111/nep.12253
Cosio FG, Grande JP, Wadei H, Larson TS, Griffin MD, Stegall MD (2005) Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant 5:2464–2472. https://doi.org/10.1111/j.1600-6143.2005.01050.x
Stegall MD, Park WD, Larson TS, Gloor JM, Cornell LD, Sethi S, Dean PG, Prieto M, Amer H, Textor S, Schwab T, Cosio FG (2011) The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant 11:698–707. https://doi.org/10.1111/j.1600-6143.2010.03312.x
Hergesell O, Felten H, Andrassy K, Kühn K, Ritz E (1998) Safety of ultrasound-guided percutaneous renal biopsy-retrospective analysis of 1090 consecutive cases. Nephrol Dial Transplant 13:975–977. https://doi.org/10.1093/ndt/13.4.975
Hassler J, Tanriover B, Ariyamutu V, Burguete D, Hendricks AR, Torrealba JR (2019) 2013 Banff criteria for acute antibody-mediated rejection are superior to 2007 Banff criteria in the diagnosis and assessment of renal allograft outcomes. Transplant Proc 51:1791–1795. https://doi.org/10.1016/j.transproceed.2019.04.060
Chua A, Cramer C, Moudgil A, Martz K, Smith J, Blydt-Hansen T, Neu A, Dharnidharka VR (2019) Kidney transplant practice patterns and outcome benchmarks over 30 years: the 2018 report of the NAPRTCS. Pediatr Transplant 23:e13597. https://doi.org/10.1111/petr.13597
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Odum, J.D., Kats, A., VanSickle, J.S. et al. Characterizing the frequency of modifiable histological changes observed on surveillance biopsies in pediatric kidney allograft recipients. Pediatr Nephrol 35, 2173–2182 (2020). https://doi.org/10.1007/s00467-020-04624-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04624-1