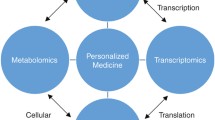
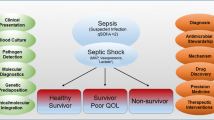
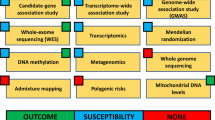
Abstract
Acute kidney injury (AKI) is common in critically ill children and adults, and sepsis-associated AKI (SA-AKI) is the most frequent cause of AKI in the ICU. To date, no mechanistically targeted therapeutic interventions have been identified. High-throughput “omic” technologies (e.g., genomics, proteomics, metabolomics, etc.) offer a new angle of approach to achieve this end. In this review, we provide an update on the current understanding of SA-AKI pathophysiology. Omic technologies themselves are briefly discussed to facilitate interpretation of studies using them. We next summarize the body of SA-AKI research to date that has employed omic technologies. Importantly, omic studies are helping to elucidate a pathophysiology of SA-AKI centered around cellular stress responses, metabolic changes, and dysregulation of energy production that underlie its clinical features. Finally, we propose opportunities for future research using clinically relevant animal models, integrating multiple omic technologies and ultimately progressing to translational human studies focusing therapeutic strategies on targeted disease mechanisms.

Similar content being viewed by others
References
Kaddourah A, Basu RK, Bagshaw SM et al (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376:11–20. https://doi.org/10.1056/NEJMoa1611391
Bouchard J, Acharya A, Cerda J et al (2015) A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol CJASN 10:1324–1331. https://doi.org/10.2215/CJN.04360514
Bagshaw SM, Uchino S, Bellomo R et al (2007) Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol CJASN 2:431–439. https://doi.org/10.2215/CJN.03681106
Hoste EAJ, Lameire NH, Vanholder RC et al (2003) Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol JASN 14:1022–1030
Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee (2008) Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care Lond Engl 12:R47. https://doi.org/10.1186/cc6863
Fitzgerald JC, Basu RK, Akcan-Arikan A et al (2016) Acute kidney injury in pediatric severe sepsis: an independent risk factor for death and new disability. Crit Care Med 44:2241–2250. https://doi.org/10.1097/CCM.0000000000002007
Weiss SL, Balamuth F, Thurm CW et al (2019) Major adverse kidney events in pediatric sepsis. Clin J Am Soc Nephrol CJASN 14:664–672. https://doi.org/10.2215/CJN.12201018
Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM (2015) Sepsis-associated acute kidney injury. Semin Nephrol 35:2–11. https://doi.org/10.1016/j.semnephrol.2015.01.002
Bellomo R, Kellum JA, Ronco C et al (2017) Acute kidney injury in sepsis. Intensive Care Med 43:816–828. https://doi.org/10.1007/s00134-017-4755-7
Fitzgerald JC, Ross ME, Thomas NJ et al (2018) Risk factors and inpatient outcomes associated with acute kidney injury at pediatric severe sepsis presentation. Pediatr Nephrol Berl Ger 33:1781–1790. https://doi.org/10.1007/s00467-018-3981-8
Stanski NL, Stenson EK, Cvijanovich NZ et al (2020) PERSEVERE biomarkers predict severe acute kidney injury and renal recovery in pediatric septic shock. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.201911-2187OC
Poukkanen M, Vaara ST, Pettilä V et al (2013) Acute kidney injury in patients with severe sepsis in Finnish intensive care units. Acta Anaesthesiol Scand 57:863–872. https://doi.org/10.1111/aas.12133
Kellum JA, Chawla LS, Keener C et al (2016) The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med 193:281–287. https://doi.org/10.1164/rccm.201505-0995OC
KDIGO AKI Writing Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138
Hotchkiss RS, Swanson PE, Freeman BD et al (1999) Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27:1230–1251
Kosaka J, Lankadeva YR, May CN, Bellomo R (2016) Histopathology of septic acute kidney injury: a systematic review of experimental data. Crit Care Med 44:e897–e903. https://doi.org/10.1097/CCM.0000000000001735
Lerolle N, Nochy D, Guérot E et al (2010) Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med 36:471–478. https://doi.org/10.1007/s00134-009-1723-x
Aslan A, van den Heuvel MC, Stegeman CA et al (2018) Kidney histopathology in lethal human sepsis. Crit Care Lond Engl 22:359. https://doi.org/10.1186/s13054-018-2287-3
Takasu O, Gaut JP, Watanabe E et al (2013) Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187:509–517. https://doi.org/10.1164/rccm.201211-1983OC
Maiden MJ, Otto S, Brealey JK et al (2016) Structure and function of the kidney in septic shock. a prospective controlled experimental study. Am J Respir Crit Care Med 194:692–700. https://doi.org/10.1164/rccm.201511-2285OC
Langenberg C, Bagshaw SM, May CN, Bellomo R (2008) The histopathology of septic acute kidney injury: a systematic review. Crit Care Lond Engl 12:R38. https://doi.org/10.1186/cc6823
Langenberg C, Gobe G, Hood S et al (2014) Renal histopathology during experimental septic acute kidney injury and recovery. Crit Care Med 42:e58–e67. https://doi.org/10.1097/CCM.0b013e3182a639da
Verma SK, Molitoris BA (2015) Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin Nephrol 35:96–107. https://doi.org/10.1016/j.semnephrol.2015.01.010
Ince C, Mayeux PR, Nguyen T et al (2016) The endothelium in sepsis. Shock Augusta Ga 45:259–270. https://doi.org/10.1097/SHK.0000000000000473
Post EH, Kellum JA, Bellomo R, Vincent J-L (2017) Renal perfusion in sepsis: from macro- to microcirculation. Kidney Int 91:45–60. https://doi.org/10.1016/j.kint.2016.07.032
Gordon AC, Mason AJ, Thirunavukkarasu N et al (2016) Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: the VANISH randomized clinical trial. JAMA 316:509–518. https://doi.org/10.1001/jama.2016.10485
Ma S, Evans RG, Iguchi N et al (2019) Sepsis-induced acute kidney injury: a disease of the microcirculation. Microcirc N Y N 1994 26:e12483. https://doi.org/10.1111/micc.12483
Murugan R, Karajala-Subramanyam V, Lee M et al (2010) Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int 77:527–535. https://doi.org/10.1038/ki.2009.502
Langenberg C, Wan L, Egi M et al (2006) Renal blood flow in experimental septic acute renal failure. Kidney Int 69:1996–2002. https://doi.org/10.1038/sj.ki.5000440
Langenberg C, Bellomo R, May C et al (2005) Renal blood flow in sepsis. Crit Care Lond Engl 9:R363–R374. https://doi.org/10.1186/cc3540
Lee S-Y, Lee Y-S, Choi H-M et al (2012) Distinct pathophysiologic mechanisms of septic acute kidney injury: role of immune suppression and renal tubular cell apoptosis in murine model of septic acute kidney injury. Crit Care Med 40:2997–3006. https://doi.org/10.1097/CCM.0b013e31825b912d
Pool R, Gomez H, Kellum JA (2018) Mechanisms of organ dysfunction in Sepsis. Crit Care Clin 34:63–80. https://doi.org/10.1016/j.ccc.2017.08.003
Emlet DR, Shaw AD, Kellum JA (2015) Sepsis-associated AKI: epithelial cell dysfunction. Semin Nephrol 35:85–95. https://doi.org/10.1016/j.semnephrol.2015.01.009
Gómez H, Kellum JA (2016) Sepsis-induced acute kidney injury. Curr Opin Crit Care 22:546–553. https://doi.org/10.1097/MCC.0000000000000356
Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA (2019) Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 96:1083–1099. https://doi.org/10.1016/j.kint.2019.05.026
Gomez H, Ince C, De Backer D et al (2014) A unified theory of Sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics and the tubular cell adaptation to injury. Shock Augusta Ga 41:3–11. https://doi.org/10.1097/SHK.0000000000000052
Gómez H, Kellum JA, Ronco C (2017) Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol 13:143–151. https://doi.org/10.1038/nrneph.2016.186
Hato T, Maier B, Syed F et al (2019) Bacterial sepsis triggers an antiviral response that causes translation shutdown. J Clin Invest 129:296–309. https://doi.org/10.1172/JCI123284
Hultström M, Becirovic-Agic M, Jönsson S (2018) Comparison of acute kidney injury of different etiology reveals in-common mechanisms of tissue damage. Physiol Genomics 50:127–141. https://doi.org/10.1152/physiolgenomics.00037.2017
Kaushal GP, Shah SV (2016) Autophagy in acute kidney injury. Kidney Int 89:779–791. https://doi.org/10.1016/j.kint.2015.11.021
Sunahara S, Watanabe E, Hatano M et al (2018) Influence of autophagy on acute kidney injury in a murine cecal ligation and puncture sepsis model. Sci Rep 8:1050. https://doi.org/10.1038/s41598-018-19350-w
Hsiao H-W, Tsai K-L, Wang L-F et al (2012) The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock Augusta Ga 37:289–296. https://doi.org/10.1097/SHK.0b013e318240b52a
Parikh SM, Yang Y, He L et al (2015) Mitochondrial function and disturbances in the septic kidney. Semin Nephrol 35:108–119. https://doi.org/10.1016/j.semnephrol.2015.01.011
Singer M (2014) The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5:66–72. https://doi.org/10.4161/viru.26907
Parikh SM (2013) Therapeutic targeting of the mitochondrial dysfunction in septic acute kidney injury. Curr Opin Crit Care 19:554–559. https://doi.org/10.1097/MCC.0000000000000038
Sun J, Zhang J, Tian J et al (2019) Mitochondria in sepsis-induced AKI. J Am Soc Nephrol JASN 30:1151–1161. https://doi.org/10.1681/ASN.2018111126
Tran M, Tam D, Bardia A et al (2011) PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121:4003–4014. https://doi.org/10.1172/JCI58662
Singer M, De Santis V, Vitale D, Jeffcoate W (2004) Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet Lond Engl 364:545–548. https://doi.org/10.1016/S0140-6736(04)16815-3
Thurau K, Boylan JW (1976) Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med 61:308–315. https://doi.org/10.1016/0002-9343(76)90365-x
Singer M (2017) Critical illness and flat batteries. Crit Care Lond Engl 21:309. https://doi.org/10.1186/s13054-017-1913-9
Sharma NK, Salomao R (2017) Sepsis through the eyes of proteomics: the progress in the last decade. Shock Augusta Ga 47:17–25. https://doi.org/10.1097/SHK.0000000000000698
Marx D, Metzger J, Pejchinovski M et al (2018) Proteomics and metabolomics for AKI diagnosis. Semin Nephrol 38:63–87. https://doi.org/10.1016/j.semnephrol.2017.09.007
Li M, Carey J, Cristiano S et al (2017) Genome-wide association of copy number polymorphisms and kidney function. PLoS One 12:e0170815. https://doi.org/10.1371/journal.pone.0170815
Cañadas-Garre M, Anderson K, Cappa R et al (2019) Genetic susceptibility to chronic kidney disease - some more pieces for the heritability puzzle. Front Genet 10:453. https://doi.org/10.3389/fgene.2019.00453
Verbitsky M, Westland R, Perez A et al (2019) The copy number variation landscape of congenital anomalies of the kidney and urinary tract. Nat Genet 51:117–127. https://doi.org/10.1038/s41588-018-0281-y
Frank AJ, Sheu C-C, Zhao Y et al (2012) BCL2 genetic variants are associated with acute kidney injury in septic shock*. Crit Care Med 40:2116–2123. https://doi.org/10.1097/CCM.0b013e3182514bca
Vilander LM, Kaunisto MA, Vaara ST et al (2017) Genetic variants in SERPINA4 and SERPINA5, but not BCL2 and SIK3 are associated with acute kidney injury in critically ill patients with septic shock. Crit Care Lond Engl 21:47. https://doi.org/10.1186/s13054-017-1631-3
Larach DB, Engoren MC, Schmidt EM, Heung M (2018) Genetic variants and acute kidney injury: a review of the literature. J Crit Care 44:203–211. https://doi.org/10.1016/j.jcrc.2017.11.019
Ferreyra GA, Elinoff JM, Demirkale CY et al (2014) Late multiple organ surge in interferon-regulated target genes characterizes staphylococcal enterotoxin B lethality. PLoS One 9:e88756. https://doi.org/10.1371/journal.pone.0088756
Basu RK, Standage SW, Cvijanovich NZ et al (2011) Identification of candidate serum biomarkers for severe septic shock-associated kidney injury via microarray. Crit Care Lond Engl 15:R273. https://doi.org/10.1186/cc10554
Wong HR, Cvijanovich NZ, Anas N et al (2015) A multibiomarker-based model for estimating the risk of septic acute kidney injury. Crit Care Med 43:1646–1653. https://doi.org/10.1097/CCM.0000000000001079
Ge Q-M, Huang C-M, Zhu X-Y et al (2017) Differentially expressed miRNAs in sepsis-induced acute kidney injury target oxidative stress and mitochondrial dysfunction pathways. PLoS One 12:e0173292. https://doi.org/10.1371/journal.pone.0173292
Wu F, Dong X-J, Li Y-Y et al (2015) Identification of phosphorylated MYL12B as a potential plasma biomarker for septic acute kidney injury using a quantitative proteomic approach. Int J Clin Exp Pathol 8:14409–14416
Hinkelbein J, Böhm L, Braunecker S et al (2017) Decreased tissue COX5B expression and mitochondrial dysfunction during sepsis-induced kidney injury in rats. Oxidative Med Cell Longev N Y 2017. https://doi.org/10.1155/2017/8498510
Matejovic M, Tuma Z, Moravec J et al (2016) Renal proteomic responses to severe Sepsis and surgical trauma: dynamic analysis of porcine tissue biopsies. SHOCK 46:453–464. https://doi.org/10.1097/SHK.0000000000000613
Thongboonkerd V, Chiangjong W, Mares J et al (2009) Altered plasma proteome during an early phase of peritonitis-induced sepsis. Clin Sci 116:721–730. https://doi.org/10.1042/CS20080478
Hashida T, Nakada T-A, Satoh M et al (2017) Proteome analysis of hemofilter adsorbates to identify novel substances of sepsis: a pilot study. J Artif Organs 20:132–137. https://doi.org/10.1007/s10047-016-0936-3
Gong Y, Chen N, Wang F-Q et al (2009) Serum proteome alteration of severe sepsis in the treatment of continuous renal replacement therapy. J Artif Organs 24:3108–3114. https://doi.org/10.1093/ndt/gfp231
Holly MK, Dear JW, Hu X et al (2006) Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int 70:496–506. https://doi.org/10.1038/sj.ki.5001575
Metzger J, Kirsch T, Schiffer E et al (2010) Urinary excretion of twenty peptides forms an early and accurate diagnostic pattern of acute kidney injury. Kidney Int 78:1252–1262. https://doi.org/10.1038/ki.2010.322
Carrick E, Vanmassenhove J, Glorieux G et al (2016) Development of a MALDI MS-based platform for early detection of acute kidney injury. Proteomics Clin Appl 10:732–742. https://doi.org/10.1002/prca.201500117
Maddens B, Ghesquière B, Vanholder R et al (2012) Chitinase-like proteins are candidate biomarkers for sepsis-induced acute kidney injury. Mol Cell Proteomics MCP 11:M111.013094. https://doi.org/10.1074/mcp.M111.013094
Waltz P, Carchman E, Gomez H, Zuckerbraun B (2016) Sepsis results in an altered renal metabolic and osmolyte profile. J Surg Res 202:8–12. https://doi.org/10.1016/j.jss.2015.12.011
Izquierdo-Garcia JL, Nin N, Cardinal-Fernandez P et al (2018) Identification of novel metabolomic biomarkers in an experimental model of septic acute kidney injury. Am J Physiol Renal Physiol. https://doi.org/10.1152/ajprenal.00315.2018
Li P, Liao S-T, Wang J-S et al (2017) Protection by Huang-Lian-Jie-Du decoction and its constituent herbs of lipopolysaccharide-induced acute kidney injury. FEBS Open Bio 7:221–236. https://doi.org/10.1002/2211-5463.12178
Rodrigues FAP, Santos ADDC, de Medeiros PHQS et al (2018) Gingerol suppresses sepsis-induced acute kidney injury by modulating methylsulfonylmethane and dimethylamine production. Sci Rep 8:12154. https://doi.org/10.1038/s41598-018-30522-6
Langley RJ, Tsalik EL, van Velkinburgh JC et al (2013) An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med 5:195ra95. https://doi.org/10.1126/scitranslmed.3005893
Langley RJ, Tipper JL, Bruse S et al (2014) Integrative “omic” analysis of experimental bacteremia identifies a metabolic signature that distinguishes human sepsis from systemic inflammatory response syndromes. Am J Respir Crit Care Med 190:445–455. https://doi.org/10.1164/rccm.201404-0624OC
Cambiaghi A, Díaz R, Martinez JB et al (2018) An innovative approach for the integration of proteomics and metabolomics data in severe septic shock patients stratified for mortality. Sci Rep 8:6681. https://doi.org/10.1038/s41598-018-25035-1
Huang H, van Dullemen LFA, Akhtar MZ et al (2018) Proteo-metabolomics reveals compensation between ischemic and non-injured contralateral kidneys after reperfusion. Sci Rep 8:8539. https://doi.org/10.1038/s41598-018-26804-8
Hato T, Zollman A, Plotkin Z et al (2018) Endotoxin preconditioning reprograms S1 tubules and macrophages to protect the kidney. J Am Soc Nephrol JASN 29:104–117. https://doi.org/10.1681/ASN.2017060624
Jha AK, Huang SC-C, Sergushichev A et al (2015) Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42:419–430. https://doi.org/10.1016/j.immuni.2015.02.005
Acknowledgments
The authors would like to thank Dr. Prasad Devarajan and Dr. Hector Wong for helping conceive and develop this work and for their insightful editorial input.
Funding
SWS receives grant support from the National Heart, Lung, and Blood Institute, National Institutes of Health (1K08HL133377-01A1).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the review. Denise Hasson and Steve Standage wrote the initial draft of the manuscript which was edited, revised, and ultimately approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasson, D., Goldstein, S.L. & Standage, S.W. The application of omic technologies to research in sepsis-associated acute kidney injury. Pediatr Nephrol 36, 1075–1086 (2021). https://doi.org/10.1007/s00467-020-04557-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04557-9