Abstract
Background
Complete mesocolic excision (CME) or D3 lymphadenectomy led to survival benefits for locally advanced right colon cancer, but with vague definitions in anatomy and debated surgical hazard in clinic. Aiming to achieve a precise definition of it in anatomy, we proposed laparoscopic right hemicolectomy (D3 + CME) as a novel procedure for colon cancer. However, the surgical and oncological results of this procedure in clinic were uncertain.
Methods
We performed a cohort study involving prospective data collected from a single-center in China. Data from all patients who underwent right hemicolectomy between January 2014 and December 2018 were included. We compared the surgical and oncological outcomes between D3 + CME and conventional CME.
Results
After implementation of exclusion criteria, a total of 442 patients were included. D3 + CME group performed better in lymph nodes harvested (25.0 [17.0, 33.8] vs. 18.0 [14.0, 25.0], P < 0.001) and the proportion of intraoperative blood loss ≥ 50 mL (31.7% vs. 51.8%, P < 0.001); no significant difference was observed in the complication rates between two groups. Kaplan–Meier analysis demonstrated that a better cumulative 5-year disease-free survival (91.3% vs. 82.2%, P = 0.026) and a better cumulative 5-year overall survival (95.2% vs. 86.1%, P = 0.012) were obtained in the D3 + CME group. Multivariate COX regression revealed that D3 + CME was an independent protective factor for disease-free survival (P = 0.026).
Conclusion
D3 + CME could improve surgical and oncological outcomes simultaneously for right colon cancer compared to conventional CME. Large-scale randomized controlled trials were further required to confirm this conclusion, if possible.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer is a threatening disease for human health worldwide, ranking third and second in terms of incidence and mortality among cancers, respectively [1]. Radical surgery remains the main treatment for locally advanced colorectal cancer [2].
Two surgical procedures have emerged in recent years: D3 lymphadenectomy advocated by Eastern countries [3] and complete mesocolic excision (CME) proposed by Western countries [4]. The former was defined as complete dissection of all regional lymph nodes (till D3 area) and transection of the colon 10 cm distal to the tumor [2], while the later emphasized the importance of removing mesocolon at the embryological planes and preserving the mesocolic integrity [4] (Figure S1). Both these two procedures seemed to be equally effective in improving the long-term prognosis of patients with colon cancer [5,6,7,8,9,10,11]. However, the surgical risks of both are controversial to date [6, 12,13,14,15,16]; the oncological benefits of D3 or CME surgery still lack high-quality evidence of randomized controlled trials [17,18,19,20,21,22]. In the paper published recently, “CME”, “CVL”, “D3” and other terms were used to describe or mark the surgical methods [7, 10, 23], but different studies were inconsistent as regard of procedure names and resection boundary [24]. The lack of a precise surgical definition based on anatomy may result in misinterpretation for the research results [25], which might be the major reason of various survival outcomes observed in different studies [26]. Briefly, it seemed that smeared-out definitions and debated surgical hazards were common characteristics of these procedures.
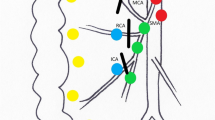
In order to make the resection boundary more precise, we proposed a novel procedure, i.e., laparoscopic right hemicolectomy (D3 + CME) [27], for colon cancer in 2017. Briefly, D3 + CME means complete excision of mesocolon in D3 boundary. In detail, right-sided mesocolon, as an envelop-like structure, is raised up from its bed to the root of the mesentery which is located at the bifurcation of branch vessels of superior mesenteric artery (SMA) and superior mesenteric vein (SMV). Then the branch vessels are ligated at their bifurcation, and the whole sample containing lymph nodes is removed as a bloc at last. In this procedure, “D3” means the distance from the primary lesion to the D3 area lymph nodes, “CME” means keeping the integrity of the mesentery as complete as possible. In this way, the procedure gets a definition with geometrical boundary (Fig. 1 and Figure S1) and ensures the integrity of mesentery as much as possible [28]. In recent years, live surgeries of D3 + CME have been conducted many times in Europe and China to show the feasibility of such a novel technique, and the replications of it have been completed by other centers. However, people wondered if such a procedure with a precise definition was safe or necessary. Here, we present the results of a prospective single-center cohort study from our team.
Schematic diagram of surgery in right-sided colon cancer. Schematic to show the procedure of a D3, b CME and c D3 + CME surgery, respectively. d Three-dimensional envelop-like structure of D3 + CME resection boundary. D3 + CME, D3 lymphadenectomy plus complete mesocolic excision; CME, complete mesocolic excision; D3, D3 lymphadenectomy; SMA, superior mesocolic artery; SMV, superior mesocolic vein. MCA, middle colic artery; MCV, middle colic vein. The brown parts indicated the tumor, the red parts indicated arteries, the bule parts indicated veins and the green parts indicated lymphatic system
Methods
Patients
This study included patients underwent right hemicolectomy from January 1, 2014 to December 31, 2018 in a single center (Department of Gastrointestinal Surgery, Tongji Hospital, Wuhan, China). Inclusion criteria: (1) Patients with right hemicolectomy; (2) Tumor sited in the caecum, ascending colon, hepatic flexure, or proximal transverse colon. Exclusion criteria: (1) Patients with emergency surgery or open surgery; (2) Patients with non-adenocarcinoma pathological results; (3) Patients with palliative resection or distant metastases. Information of patient demographic characteristics, preoperative assessment, intraoperative conditions, and postoperative outcomes was extracted in a prospective database. Preoperative anesthetic risk was assessed by American Society of Anesthesiologists (ASA) classification [29] for each patient. Charlson Comorbidity Index (CCI) [30] was calculated for each patient according to patient baseline information. Postoperative tumor pathological staging was performed according to American Joint Committee on Cancer (AJCC) 8th edition. All the participants provided informed written consent to participate in this study. Ethical approval for this study was granted by the hospital's ethics committee.
Procedure
Laparoscopic D3 + CME surgery was performed by the inventor of this procedure in our center, and the surgical procedure was described in our previous research (attached with a surgery video) in detail [27]. Briefly, D3 + CME means that CME is made in D3 field, which requires the surgeon to identify three “Tri-junction” points and ensure the relative integrity of the mesentery when dissecting the fusion plane of different mesentery. Following this principle, the ileocolic mesentery and the middle colic mesentery, the right gastroepiploic mesentery and the middle colic mesentery, and the right mesocolon and its mesenteric bed are sequentially separated. The lateral boundary of D3 + CME is the fusion edge between the parietal peritoneum and the right mesocolon, the upper boundary is the fusion site of the right gastroepiploic mesentery and the middle colic mesentery, the lower boundary is the fusion site of the ileocolic mesentery and the ileum mesentery, the posterior boundary is the fusion plane of the right mesocolon and its mesentery bed (retroperitoneum, duodenum, pancreas, etc.), and the medial boundary is the left edge of the SMA. Figure S2 showed the sample of laparoscopic view of D3 + CME. While the procedures performed in the control group completed CME without following the D3 + CME concept, and was performed by other surgeons experienced in laparoscopic right hemicolectomy.
Surgical outcome
Complications were graded by using the Clavien-Dindo classification [31] and included chyle leak, pulmonary infection, abdominal infection, wound infection, anastomotic fistula, intestinal obstruction, postoperative abdominal pain, and other complications. Intraoperative bleeding volume was estimated and recorded in the database by the anesthesiologist. Lymph nodes retrieving were performed by specialized pathologists.
Patients Follow-up and Long-term Outcome
Follow-up data were registered prospectively in the medical records of the departments. In principle, follow-up was conducted three months interval for the first year after surgery, 6 months interval for the second year, and annually thereafter. The postoperative surveillance consisted of physical examination, quality of life, CT scan, blood tumor biomarker (including CEA, carbohydrate antigen (CA) 19–9 and CA72-(4), and the colonoscopy results. The primary long-term outcomes were cumulative disease-free survival (DFS) and overall survival (OS).
Statistical analysis
Data normality was determined by the Kolmogorov–Smirnov test for continuous variables, with normally distributed data expressed as mean (SD) and non-normally distributed data expressed as median [IQR]; categorical variables were expressed as n (%). The t-test or Man–Whitney U test was used for continuous variables, and the chi-square test or Fisher exact test was used for categorical variables. Kaplan–Meier (KM) method and log-rank test was used for survival data analyzing and survival curves plotting. Univariate and multivariate COX regression were used to determine risk factors for disease-free survival. P value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS Statistics for Windows v26.0 (Armonk, NY: IBM Corp).
Results
Patients’ characteristics
From January 1, 2014 to December 31, 2018, a total of 652 patients underwent right hemicolectomy were enrolled. One hundred and sixteen of them were excluded because they underwent emergency surgery or open surgery; forty-three patients with benign pathological findings and 9 patients with other tumors were excluded from the analysis; fifty-one patients with distant metastases or palliative resections were also excluded. The final patients included in the analysis were 104 patients in the D3 + CME group and 338 patients in the conventional group (Fig. 2).
The characteristics of the patients' baseline and pathology were shown in Table 1 and Table 2. All baseline characteristics as well as pathological results between the two groups were comparable.
Surgical outcomes
In the short-term outcomes (Table 3), the operative time was significantly longer in the D3 + CME group compared to the control group (256.0 [230.0, 288.0] vs. 236.5 [209.0, 262.3], P < 0.001). The proportion of patients with estimated intraoperative bleeding of more than 50 ml was significantly less in the D3 + CME group compared to the control group (31.7% vs. 51.8%, P < 0.001). There was also a significant increase in the number of lymph nodes retrieved in the D3 + CME group (25.0 [17.0, 33.8] vs. 18.0 [14.0, 25.0], P < 0.001). And in terms of hospital stay, there was a prolongation in the D3 + CME group (10.0 [9.0, 12.0] vs. 10.0 [9.0, 11.0], P = 0.026). No significant differences were found in the comparison of the conversion rate as well as the complication rate between the two groups.
Survival analysis
The median follow-up time was 46.5 (range 0.5–83.3) months for patients in D3 + CME group and 45.0 (range 0.4–85.7) months for the control group. The KM analysis (Fig. 3a, b) revealed that among all AJCC stage I-III patients, patients in the D3 + CME group had a better DFS than those in the control group (91.3% vs. 82.2%, P = 0.026), and the same findings applied to OS (95.2% vs. 86.1%, P = 0.012). In addition, after excluding patients with AJCC stage I (Fig. 3c, d), subgroup analysis showed that patients in the D3 + CME group still had a more favorable prognosis (DFS: 90.5% vs. 81.5%, P = 0.038 and OS 94.7% vs. 84.9%, P = 0.012, respectively). Stage I, II and III survival condition were demonstrated in Figure S3, respectively.
We identified independent factors associated with DFS by univariate and multivariate COX regression analysis (Table 4). Age (P = 0.025), pN stage (P < 0.001) and tumor differentiation classification (P = 0.028) were independent risk factors for DFS, while D3 + CME (P = 0.026) was an independent protective factor for DFS.
Discussion
In this study, we expressed the resection boundary and key points of D3 + CME through geometrical boundary, avoiding a confusing concept to some extent. D3 + CME is not simple combination of D3 lymphadenectomy and CME, but integrates CME principle into the D3 lymph node dissection process [32], with clear three-dimensional geometric boundary described by resected colon length, distance from primary lesion to the roots of main branch vessels including D3 area lymph nodes, and envelop-like structure as peripheral boundary (Fig. 1d).
According to the results presented in our study, D3 + CME performed better in clinical outcomes: (1) Less surgical hazard: bleeding volume during operation reduced without complication rate increasing (Table 3). (2) Oncological benefit: the number of lymph nodes harvested increased, and 5-year cumulative OS and DFS improved (Fig. 3). Moreover, as a result of its clear definition, this procedure was of good feasibility and stability, as we have repeatedly demonstrated in domestic and international symposium by LIVE SURGERY; such an operation was also replicated by surgeons in other centers (personal communication), which suggested that it was fitted to popularization. In summary, the procedure, laparoscopic right hemicolectomy (D3 + CME), not only had a clearer geometric definition, but also could improve surgical and oncological outcomes simultaneously.
As we know, people have conducted trials to compare D3 surgery with D2 surgery [23] or CME with non-CME [26], but various conclusions about the oncological survival were drawn in different studies [25]. We compared the control group (CME) data in this study with the largest CME survival data that have ever reported [26], they seemed to be consistent with each other, which meant that the control group in this study was comparable and convincing (Table S1). As the results demonstrated, we compared the D3 + CME group with the control group of this study, and found that the survival of D3 + CME group was much better than conventional CME (Fig. 3).
Interestingly, data revealed that the survival advantage of D3 + CME also existed in those cases with pN0 stage (Figure S4). Such a result may not be completely explained by radical lymph nodes dissection. In previous studies, we proposed the hypothesis of “ Metastasis V” [33] which is different from the well-known direct invasion, lymphatic metastasis, hematogenous metastasis and implantation metastasis. Subsequently, we found that such a metastasis approach was independent of lymphatic metastasis in gastrointestinal cancer [34, 35], and preserving the integrity of the mesentery will minimize the scattered cancer cells in the mesenteric adipose tissue “leaking out” into the abdominal serous cavity [36]. This may explain, to some extent, the oncological advantages of D3 + CME in the cases without lymph node metastasis and provided another evidence supporting hypothesis of “Metastasis V”. Additionally, removal of occult positive lymph nodes which cannot be detected by conventional examination could also be a possible reason for this result.
We would like to propose an anatomical model here to explain the superiority of D3 + CME: “mesentery in broad sense”. Mesentery in broad sense (MBS) is an envelop-like structure that the proper facial membrane (and serous membrane in serous cavity) enclose the organ (or tissue) and its feeding structure (vascular system), suspending to and leading to the posterior wall of the body. MBS structures lie on (or are buried in) their mesenteric beds, and fuse with adjacent MBS structures or serous cavity wall. Moreover, MBS could be raised up to the root from its bed. Disruption of the envelop-like structure would lead to bleeding and cancer cell leaking, resulting in surgical risk and local recurrence.
There are four “weak” parts in MBS structure of the right mesocolon which were vague and imprecise, which could explain potential surgical hazard and local recurrence risk in conventional CME group: (1) The middle colic mesentery part is fused with the right gastroepiploic mesentery and can be regarded as a mesenteric bed for each other. In D3 + CME procedure, the middle colic mesentery could be stripped up from its bed (the right gastroepiploic mesentery) to the root [27, 37], avoiding any damage leading to bleeding or cancer cell leaking out from the envelop-like structure to the serous cavity. However, this anatomical feature had not been understood well and was difficult to identify in laparotomy, especially among surgeons specializing in colorectal surgery (Figure S2c). (2) The ileocolic mesentery is clustered. It could be easily raised up from its mesenteric bed, that is, the posterior wall of the serous cavity. In fact, there is a “thin” boundary between the ileocolic mesentery and the ileum mesentery, and the clustered ileocolic mesentery should be kept as complete as possible to avoid damage (Figure S2d). (3) The middle colic mesentery and ileocolic mesentery merge to form the “central part” of the right mesocolon, which can be stripped out from the anterior surface of SMV and SMA (Figure S2a and S2b). In this way, the central part of the right mesocolon can be removed completely without bleeding or adjacent structures (such as lacteal, visceral nerves and so on) damage, and more D3 area lymph nodes can be harvested, and the branches can be ligated at their bifurcation precisely. (4) In our opinion, the real envelop-like structure of the right mesocolon lies on the posterior wall of the serous cavity and fuses with it; the peritoneal “reflex” along the ascending colon is the only secondary “illusion” or the edge of the right mesocolon and its mesenteric bed. Inadequate understanding of these features or regarding the right mesocolon is “out of” the serous cavity would lead to wrong “plane anatomy” or envelop-like structure destruction. It is the MBS structure in which local life events occur that clarifies the boundary and definition of D3 + CME procedure.
In the framework of this geometrical model mentioned above, many phenomena and results seem to be explained well. Firstly, D3 + CME follows the geometrical boundary for surgical mobilization and resection, avoiding damage to adjacent structures during operation. Therefore, the seemingly expanded boundary of surgery would not increase the incidence of complications. Secondly, it keeps the integrity of the MBS structure as much as possible, protecting the blood vessels and lymphatic system in it from being damaged, and preventing scattered cancer cells from leaking out, resulting in less bleeding and better long-term outcomes. In addition, as a result of precise surgical boundary, D3 + CME is of good repeatability. In contrast, without clear understandings of the MBS structure and geometrical descriptions, conventional CME/D3 surgery might disrupt the MBS structure (destroying the integrity of the envelope or resulting in part of MBS structure residual) when mobilizing along the SMV and SMA, which would be a potential source of bleeding or cancer cell leaking (Table 3 and Fig. 3).
Based on the MBS model, we proposed an anatomical model named the proximal segment of dorsal mesogastrium (PSDM) in gastric cancer research [37], and subsequently proposed the MBS-based surgery named D2 + CME [38]; we then demonstrated the advantages of that procedure in surgical and oncological outcomes [36, 39,40,41]. The study D3 + CME for colon cancer here is amazingly consistent with D2 + CME for gastric cancer. This indicated that envelop-like structure of MBS model was of good compatibility to some extent. Surgical risk and oncological prognosis, as two major concerning problems that have puzzled surgeons for a long period, seemed to be solved in this geometric model by applying natural anatomic boundary. Based on the guidance of this model, we expected more and more envelop-like structures of MBS or “extra-bowel mesentery” would be identified, explored and utilized [42].
This current study suffered from a certain of limitations. Lower average BMI than western population, single-institutional design and relatively small sample size, which should be improved by well-designed randomized controlled trials in the following work.
In conclusion, laparoscopic right hemicolectomy (D3 + CME) with a more precise geometrical boundary and definition, seemed to be not only feasible, but also improving surgical hazard and oncological survival at same time.
Change history
18 May 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00464-023-10138-2
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin 71:209–249
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K, Japanese Society for Cancer of the Colon Rectum (2020) Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25:1–42
Dennosuke Jinnai (1983) General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Japanese J Surg 13:574–598
Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation–technical notes and outcome. Colorectal Dis 11:354–364
Crane J, Hamed M, Borucki JP, El-Hadi A, Shaikh I, Stearns AT (2021) Complete mesocolic excision versus conventional surgery for colon cancer: a systematic review and meta-analysis. Colorectal Dis 23:1670–1686
Kong JC, Prabhakaran S, Choy KT, Larach JT, Heriot A, Warrier SK (2021) Oncological reasons for performing a complete mesocolic excision: a systematic review and meta-analysis. ANZ J Surg 91:124–131
Ow ZGW, Sim W, Nistala KRY, Ng CH, Koh FH, Wong NW, Foo FJ, Tan KK, Chong CS (2021) Comparing complete mesocolic excision versus conventional colectomy for colon cancer: a systematic review and meta-analysis. Eur J Surg Oncol 47:732–737
De Simoni O, Barina A, Sommariva A, Tonello M, Gruppo M, Mattara G, Toniato A, Pilati P, Franzato B (2021) Complete mesocolic excision versus conventional hemicolectomy in patients with right colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 36:881–892
Balciscueta Z, Balciscueta I, Uribe N, Pellino G, Frasson M, Garcia-Granero E, Garcia-Granero A (2021) D3-lymphadenectomy enhances oncological clearance in patients with right colon cancer. results of a meta-analysis. Eur J Surg Oncol 47:1541–1551
Ferri V, Vicente E, Quijano Y, Duran H, Diaz E, Fabra I, Malave L, Agresott R, Isernia R, Cardinal-Fernandez P, Ruiz P, Nola V, de Nobili G, Ielpo B, Caruso R (2021) Right-side colectomy with complete mesocolic excision vs conventional right-side colectomy in the treatment of colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis 36:1885–1904
West NP, Kobayashi H, Takahashi K, Perrakis A, Weber K, Hohenberger W, Sugihara K, Quirke P (2012) Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 30:1763–1769
Freund MR, Edden Y, Reissman P, Dagan A (2016) Iatrogenic superior mesenteric vein injury: the perils of high ligation. Int J Colorectal Dis 31:1649–1651
Prevost GA, Odermatt M, Furrer M, Villiger P (2018) Postoperative morbidity of complete mesocolic excision and central vascular ligation in right colectomy: a retrospective comparative cohort study. World J Surg Oncol 16:214
Bertelsen CA, Neuenschwander AU, Jansen JE, Wilhelmsen M, Kirkegaard-Klitbo A, Tenma JR, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Iversen ER, Kristensen B, Gögenur I (2015) Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 16:161–168
Bertelsen CA, Neuenschwander AU, Jansen JE, Kirkegaard-Klitbo A, Tenma JR, Wilhelmsen M, Rasmussen LA, Jepsen LV, Kristensen B, Gogenur I, Copenhagen Complete Mesocolic Excision Study, Danish Colorectal Cancer G (2016) Short-term outcomes after complete mesocolic excision compared with ‘conventional’ colonic cancer surgery. Br J Surg 103:581–589
Sammour T, Malakorn S, Thampy R, Kaur H, Bednarski BK, Messick CA, Taggart M, Chang GJ, You YN (2020) Selective central vascular ligation (D3 lymphadenectomy) in patients undergoing minimally invasive complete mesocolic excision for colon cancer: optimizing the risk-benefit equation. Colorectal Dis 22:53–61
Mazzarella G, Muttillo EM, Picardi B, Rossi S, Muttillo IA (2021) Complete mesocolic excision and D3 lymphadenectomy with central vascular ligation in right-sided colon cancer: a systematic review of postoperative outcomes, tumor recurrence and overall survival. Surg Endosc 35:4945–4955
Son GM, Lee IY, Lee YS, Kye BH, Cho HM, Jang JH, Kim CN, Lee KY, Lee SH, Kim JG, Korean Laparoscopic Colorectal Surgery Study G (2021) Is laparoscopic complete mesocolic excision and central vascular ligation really necessary for all patients with right-sided colon cancer? Ann Coloproctol 37:434–444
Zizzo M, Zanelli M, Sanguedolce F, Castro Ruiz C, Biolchini F, Giunta A (2021) Complete mesocolic excision (CME) and D3-lymphadenectomy (D3) for right-sided colon cancers: a potentially prognostic surgical approach. Surg Today 51:1723–1724
Emmanuel A, Haji A (2016) Complete mesocolic excision and extended (D3) lymphadenectomy for colonic cancer: is it worth that extra effort? a review of the literature. Int J Colorectal Dis 31:797–804
Sahara K, Watanabe J, Ishibe A, Goto K, Takei S, Suwa Y, Suwa H, Ota M, Kunisaki C, Endo I (2021) Optimal extent of central lymphadenectomy for right-sided colon cancers: is lymphadenectomy beyond the superior mesenteric vein meaningful? Surg Today 51:268–275
Gouvas N, Agalianos C, Papaparaskeva K, Perrakis A, Hohenberger W, Xynos E (2016) Surgery along the embryological planes for colon cancer: a systematic review of complete mesocolic excision. Int J Colorectal Dis 31:1577–1594
Storli KE, Sondenaa K, Furnes B, Nesvik I, Gudlaugsson E, Bukholm I, Eide GE (2014) Short term results of complete (D3) vs. standard (D2) mesenteric excision in colon cancer shows improved outcome of complete mesenteric excision in patients with TNM stages I-II. Tech Coloproctol 18:557–564
Tejedor P, Francis N, Jayne D, Hohenberger W, Khan J, on behalf the CMEPWG (2022) Consensus statements on complete mesocolic excision for right-sided colon cancer-technical steps and training implications. Surg Endosc 36:5595–5601
Sica GS, Vinci D, Siragusa L, Sensi B, Guida AM, Bellato V, Garcia-Granero A, Pellino G (2022) Definition and reporting of lymphadenectomy and complete mesocolic excision for radical right colectomy: a systematic review. Surg Endosc 37:846–861
Benz SR, Feder IS, Vollmer S, Tam Y, Reinacher-Schick A, Denz R, Hohenberger W, Lippert H, Tannapfel A, Stricker I (2022) Complete mesocolic excision for right colonic cancer: prospective multicentre study. Br J Surg 110:98–105
Xie D, Yu C, Gao C, Osaiweran H, Hu J, Gong J (2017) An optimal approach for laparoscopic D3 lymphadenectomy plus complete mesocolic excision (D3+CME) for right-sided colon cancer. Ann Surg Oncol 24:1312–1313
Shen J, Xie D, Tong Y, Gong J (2017) The length and complexity of mesentery are related to the locoregional recurrence of the carcinoma in gut. Med Hypotheses 103:133–135
Keats AS (1978) The ASA classification of physical status–a recapitulation. Anesthesiology 49:233–236
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies development and validation. J Chron Dis 40:373–383
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibanes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–196
Kim NK, Kim YW, Han YD, Cho MS, Hur H, Min BS, Lee KY (2016) Complete mesocolic excision and central vascular ligation for colon cancer: principle, anatomy, surgical technique, and outcomes. Surg Oncol 25:252–262
Xie D, Osaiweran H, Liu L, Wang X, Yu C, Tong Y, Hu J, Gong J (2013) Mesogastrium: a fifth route of metastasis in gastric cancer? Med Hypotheses 80:498–500
Xie D, Liu L, Osaiweran H, Yu C, Sheng F, Gao C, Hu J, Gong J (2015) Detection and characterization of metastatic cancer cells in the mesogastrium of gastric cancer patients. PLoS ONE 10:e0142970
Luo XL, Xie DX, Wu JX, Wu AD, Ge ZQ, Li HJ, Hu JB, Cao ZX, Gong JP (2017) Detection of metastatic cancer cells in mesentery of colorectal cancer patients. World J Gastroenterol 23:6315–6320
Xie D, Wang Y, Shen J, Hu J, Yin P, Gong J (2018) Detection of carcinoembryonic antigen in peritoneal fluid of patients undergoing laparoscopic distal gastrectomy with complete mesogastric excision. Br J Surg 105:1471–1479
Xie D, Gao C, Lu A, Liu L, Yu C, Hu J, Gong J (2015) Proximal segmentation of the dorsal mesogastrium reveals new anatomical implications for laparoscopic surgery. Sci Rep 5:16287
Xie D, Yu C, Liu L, Osaiweran H, Gao C, Hu J, Gong J (2016) Short-term outcomes of laparoscopic D2 lymphadenectomy with complete mesogastrium excision for advanced gastric cancer. Surg Endosc 30:5138–5139
Shen J, Cao B, Wang Y, Xiao A, Qin J, Wu J, Yan Q, Hu Y, Yang C, Cao Z, Hu J, Yin P, Xie D, Gong J (2018) Prospective randomized controlled trial to compare laparoscopic distal gastrectomy (D2 lymphadenectomy plus complete mesogastrium excision, D2 + CME) with conventional D2 lymphadenectomy for locally advanced gastric adenocarcinoma: study protocol for a randomized controlled trial. Trials. https://doi.org/10.1186/s13063-018-2790-5
Xie D, Shen J, Liu L, Cao B, Wang Y, Qin J, Wu J, Yan Q, Hu Y, Yang C, Cao Z, Hu J, Yin P, Gong J (2021) Complete mesogastric excision for locally advanced gastric cancer: short-term outcomes of a randomized clinical trial. Cell Rep Med 2:100217
Zhao D, Deng J, Cao B, Shen J, Liu L, Xiao A, Yin P, Xie D, Gong J (2022) Short-term outcomes of D2 lymphadenectomy plus complete mesogastric excision for gastric cancer: a propensity score matching analysis. Surg Endosc 36:5921–5929
Zhu TY, Deng XM, Wang GJ, Gao BL, Li RX, Zhang YF, Wang JT (2022) En bloc mesoesophageal esophagectomy through thoracoscopy combined with laparoscopy based on the mesoesophageal theory. Surg Endosc 36:5784–5793
Acknowledgements
This work was funded by the National Natural Science Foundation of China, No.81372324. We thank all staff of the Department of Gastrointestinal Surgery of Tongji Hospital for supporting this study. We thank Chenchen Jin, Li Zhu, Jie Shi, Jing Wu, Hanhan Hu, Kun Chen, and Xuan Dou for assistance with sample collection and follow-up. We sincerely appreciate Ping Yin for his statistical assistance. We thank Yi Li for her figure painting and Yani Wang for her language assistance in this work. We apologize to the colleagues whose work was not cited due to space constraints.
Funding
National Natural Science Foundation of China, 81372324, Jianping Gong
Author information
Authors and Affiliations
Contributions
Conceptualization, JG; methodology, JG, YT, DX, and HL; data curation and statistical analysis, XW, YT, and HL; writing—original draft, JG, XW, YT, and HL; writing—review & editing, JG; visualization, XW; supervision, YT, DX, JS, and JG; project administration, JG and YT; funding acquisition, JG.
Corresponding author
Ethics declarations
Disclosures
Drs. Jianping Gong, Xiaolin Wu, Yixin Tong, Daxing Xie, Haijie Li, and Jie Shen have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, X., Tong, Y., Xie, D. et al. Surgical and oncological outcomes of laparoscopic right hemicolectomy (D3 + CME) for colon cancer: A prospective single-center cohort study. Surg Endosc 37, 6107–6117 (2023). https://doi.org/10.1007/s00464-023-10095-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-10095-w