Abstract
Background
The field of robotic liver resection (RLR) has developed in the past decades. This technique seems to improve the access to the posterosuperior (PS) segments. Evidence of a possible advantage over transthoracic laparoscopy (TTL) is not yet available. We aimed to compare RLR to TTL for tumors located in the PS segments of the liver in terms of feasibility, difficulty scoring, and outcome.
Methods
This retrospective study compared patients undergoing robotic liver resections and transthoracic laparoscopic resections of the PS segments between January 2016 and December 2022 in a high-volume HPB center. Patients’ characteristics, perioperative outcomes, and postoperative complications were evaluated.
Results
In total, 30 RLR and 16 TTL were included. Only wedge resections were performed in the TTL group, while 43% of the patients in the RLR group had an anatomical resection (p < 0.001). The difficulty score according to the IWATE difficulty scoring system was significantly higher in the RLR group (p < 0.001). Total operative time was similar between the two groups. Complication rates, either overall or major, were comparable between the two techniques and hospital stay was significantly shorter in the RLR group. Patients in the TTL group were found to have more pulmonary complications (p = 0.01).
Conclusion
RLR may provide some advantages over TTL for the resection of tumors located in the PS segments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The field of laparoscopic liver resection (LLR) has developed in the past decades despite these procedures being technically demanding with a high risk of hemorrhage. LLR is now accepted as a useful approach for both major and minor resections [1]. LLR of posterosuperior (PS) segments, defined as segments VII and VIII in Couinaud classification [2], remains technically challenging due to difficult bleeding control and liver mobilization. The 2008 Louisville Statement [3] only recommended the laparoscopic approach as standard practice for resection of anterolateral segments. In parallel with the development of the caudal approach concept for major liver resections [4], some authors have described a lateral approach for the resection of the PS segments [5]. Despite the patient being in semi-prone position, the lack of flexible instruments remains an obstacle. Transthoracic approach facilitates minimally invasive resections involving the PS segments by placing one or two ports in the right hemithorax [6,7,8,9]. These transthoracic ports penetrate through the diaphragm into the peritoneal cavity. This approach theoretically increases the risk of postoperative thoracic complications, such as pleural effusion, pulmonary infection / injury, or tumor seeding to the thoracic wall. Another way to facilitate the access to the PS segment is the use of the Da Vinci robotic system which provides articulated instruments and a magnified 3D view of the operative field [10]. Up to date, robotic surgery is reported as non-inferior to the laparoscopic approach by the 2018 international consensus statement on liver robotic surgery [11]. The Da Vinci system seems to improve the access to the PS segments [12] yet its superiority has only been demonstrated when compared to open surgery in the cases of minor resections in the PS segments [13]. However, evidence of a possible advantage over transthoracic laparoscopy (TTL) is not yet available. The aim of the present study was to compare RLR to TTL for tumors located in the PS segments of the liver in terms of feasibility, difficulty scoring, and outcome. We therefore focused on the experience of a single surgeon practicing both techniques in a high-volume hepato-pancreatico-biliary (HPB) center expert in LLR.
Patients and methods
Study design
All patients who underwent RLR or TTL between January 2016 and December 2022 performed by a single HPB / transplant surgeon in a high-volume HPB center were reviewed. The surgeon was experienced in liver laparoscopic resection (> 350 procedures). One hundred and eighteen RLR were identified, all performed between December 2018 and December 2022. A tumor located in a PS segment was identified in 33 RLR patients, regardless of whether the tumor was benign or malignant. During the study period, 23 TTL were performed. Combined extrahepatic surgery and/or resection of more than one tumor were considered as exclusion criteria. Considering the exclusion criteria, 30 patients were included in the RLR group and 16 in the TTL group (Fig. 1). All the surgical indications were validated during a dedicated multidisciplinary meeting in presence of hepatobiliary surgeons, hepatologists, oncologists, and radiologists. Cirrhotic patients were considered eligible for surgery if hepatic venous pressure gradient was below 10 mmHg [14].
The present study derives from a larger prospectively designed, registered multicenter observational study investigating the objective and subjective determinants of the postoperative course of hepatectomy. It was deemed uninterventional by the ethical committees of participating institutions and approved by the Data Protection Authority and Health Information Protection Committee. All patients were informed that data are collected in a prospectively maintained database.
Baseline characteristics collected consisted of age, sex, body mass index (BMI; kg/m2), American Society of Anesthesiologists (ASA) physical status, abdominal surgery history, presence of underlying cirrhosis, and MELD score. Surgical parameters collected were resected segments, operative time (time from incision to wound closure), intraoperative drain placement (abdominal or thoracic), blood loss, need and duration of Pringle maneuver, and conversion to open surgery. Postoperative outcomes collected were 90-day complications (according to the Clavien–Dindo classification [15]), intensive care unit admission, length of hospital stay, and 30- and 90-day mortality. A complication of grade III or higher was considered as major. A postoperative pulmonary event was defined as the occurrence of either pleural effusion, postoperatively drained pneumothorax, or the need for intensive physiotherapy due to the persistence of non-drained pneumothorax on the postoperative day 2 chest X-ray examination.
Preoperative imaging (CT scan and / or MRI) was analyzed to assess the tumor location according to Couinaud classification [2] and the IWATE difficulty score [16] taking into account the tumor size and its proximity to a major vessel, mainly the right hepatic vein.
Tumors were classified according to their pathological type: hepatocellular carcinoma, cholangiocarcinoma, metastasis, or benign tumors, including adenoma and cystadenoma. Histological tumor margin was reported as R1 when microscopically evaluated as inferior to 1 mm.
For each resected specimen, an experienced pathologist performed a specific histological analysis of representative sections of non-neoplastic hepatic parenchyma. Fatty accumulation was considered pathological for a hepatic fat content involving 30% or more of hepatocytes. Liver fibrosis was quantified according to the METAVIR score [17].
Laparoscopic procedure
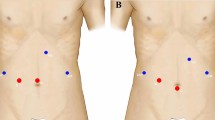
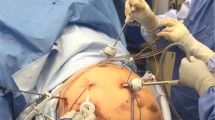
Patients were placed in a semi-prone position. Four to five abdominal ports were required (Fig. 2). Access to the peritoneal cavity was created in an open fashion on the right anterior axillary line for introduction of the 30-degree scope. The surgeon initially stood on the left side of the patient for the abdominal time. Pringle maneuver was systematically prepared. After liver mobilization, 1 or 2 transthoracic trans-diaphragmatic 10-mm ports were inserted. This procedure was always performed under vision control and while patient was not ventilated. One-lung ventilation was not necessary. The surgeon then moved to the right of the patient to perform the resection after routine ultrasonic exploration of the liver parenchyma. Parenchymal transection was performed using either the CUSA Excell (Integra) or LigaSure (Medtronic) device. Minor bleeding was controlled with bipolar forceps. Larger structures were controlled with nonabsorbable polymer locking clips or stapler as needed. After thoracic port removal, cutaneous scar was closed and the diaphragmatic breach was systematically closed using a 3–0 barbed suture to prevent diaphragmatic hernia. The created pneumothorax was aspirated with a conventional laparoscopic suction device. The right hemithorax was drained only if the previous procedure had failed. The abdomen of cirrhotic patients was systematically drained to prevent postoperative pleural effusion in case of postoperative ascites. Respiratory physiotherapy was proposed at early postoperative stage and lasted at least 5 days.
Robotic procedure
All procedures were performed using the Da Vinci X surgical system (Intuitive Surgical). The patient was placed in French position and the operating table was rotated to the left to facilitate liver mobilization. The surgical cart came from above the head. The optical port was positioned on the right anterior axillary line, 1 robotic port on the right, and 2 robotic ports on the left were added in accordance with the good practice rules for docking. A 12-mm port for the assistant was placed between the optical port and the right one. Parenchymal transection was performed using the Vessel Sealer device (Intuitive Surgical). An articulated robotic stapler was applied as needed. No transthoracic port was used. During robotic procedure, Pringle maneuver was systematically prepared, using an intracorporeal rubber loop. Thus, the main operator remains autonomous for manipulation of the hepatic pedicle clamp. Intermittent portal triad clamping was easily used as we know that bleeding can be more important during RLR than laparoscopy. In both TTL and RLR, intermittent clamping was used (15 min of ischemia followed by reperfusion for 5 min or 10–5 min in case of cirrhosis).
Aim of the study
The purpose of our study was to compare RLR and TTL for PS hepatic lesions. The main objective was to show that robotic surgery could allow performing a more difficult procedure according to difficulty score without altering the postoperative course. Secondary endpoints were the incidence of postoperative pulmonary events and the quality of the resection assessed by pathological tumor margins evaluation.
Statistical analysis
Continuous variables were expressed as median (range). They were compared using the Mann–Whitney U test, Wilcoxon’s test, or Student’s t test, as appropriate. Categorical variables were compared using Fisher’s exact test or χ2 test as appropriate. The threshold for statistical significance was set to p < 0.05. All analyses were performed using SPSS version 17 (SPSS, Chicago, IL).
Results
After application of the inclusion and exclusion criteria, a total of 46 patients were included in the study comprising 30 RLR and 16 TTL. Patients’ demographics, clinical characteristics, and indications for liver resection are summarized in Table 1. Only wedge resections were performed in the TTL group, while 43% of the patients in the RLR group had an anatomical resection (p < 0.001). In the RLR group, the right hepatic vein was resected in one patient during segmentectomy VII (allowed by the presence of an inferior right hepatic vein for the venous drainage of segment VI) and was sutured (5–0 polypropylene suture) in another patient with a metachronous metastasis in the segment VII (Fig. 3). This last procedure required a continuous clamping of 22 min. The right hepatic vein was never dissected during TTL.
Overall, the difficulty score according to the IWATE difficulty scoring system was significantly higher in the RLR group (p < 0.001). The IWATE grade of the first 41 RLR performed is presented in Fig. 4. The first PS RLR was performed after 13 RLR procedures, and the first IWATE grade 8 procedure for PS segment was the 28th. RLR resection in the PS segments reached a median IWATE grade of 7 (5–11).
The intra- and postoperative data are summarized in Table 2. Total operative time was similar between the two groups. Blood losses were higher in the RLR group (p = 0.51) with three patients in the RLR group and one in the TTL group requiring blood transfusion. In the RLR group, the first transfused patient was the first RLR one (2 units), due to both intraoperative hemorrhage and low initial hemoglobin level. In the TTL group, four units of blood were transfused to the patient whose procedure was converted to open surgery. The cause of the conversion was a hemorrhage of the inferior vena cava injured during liver mobilization, concomitant with a massive gas embolism requiring the interruption of the procedure. This patient was the ninth in the TTL group. The postoperative evolution was very favorable after emergency recompression therapy in a hyperbaric chamber, with no complication from the gas embolism, allowing discharge from hospital on day 9 and open surgery performed 6 weeks later for the treatment of the liver metastasis left behind. The patient is alive at 3 years without hepatic recurrence (though receiving second-line chemotherapy for evolving pulmonary micronodulation).
In only one patient, the conversion from RLR to open surgery occurred due to technical difficulties in liver mobilization and exposure of the operative field. The patient was obese (BMI 36) with confirmed cirrhosis and 60% steatosis. The right hepatic vein was controlled by robotic approach but for technical difficulties, conversion to open surgery was decided without any context of emergency (total blood loss of 600 mL, 400 mL at the time of conversion). During hospitalization, this patient presented bile leakage and pleural effusion justifying thoracic drainage and prolonged antibiotic therapy.
Complication rates, either overall or major, were similar between groups (Table 2). No gas embolism occurred during RLR and only four pulmonary events. In the TTL group, the total number of pulmonary event was 8 including 4 intraoperative thoracic drainages and 4 persistent postoperative pneumothoraxes on postoperative day 2 chest X-ray examination. This difference in pulmonary events between the two groups reached statistical significance (p = 0.01). Length of hospital stay and intensive care unit stay were shorter in the RLR group.
After a subgroup analysis comparing the wedge resections in the RLR group with the TTL group, the IWATE score and the proportion of segment VII / VIII are similar. Operative time was shorter using the Da Vinci (126 min [57—163]), although there were no statistically significant differences. One patient experienced severe complication (parietal bleeding, Clavien–Dindo IIIb) in the RLR subgroup. However, regarding overall complications, there was no statistical difference between the two groups. No pulmonary event was described after robotic wedge resection (p < 0.01).
There was no difference in the quality of resection (pathological R1 margin on surgical specimen). The R1 resections in the RLR group were reported for anatomical segmentectomies and involved for example contact with the right hepatic vein (anatomical resection of an HCC with close contact with the vein in a patient not fit for a right hepatectomy). In the TTL group, R1 resection was observed for one case of liver metastasis and was a parenchymal R1.
Discussion
To our knowledge, only one retrospective study with propensity score has compared RLR and laparoscopic resection in the PS segments of the liver [18]. It was published in 2016 by an Italian team and enrolled 36 RLR from two HPB centers. However, the authors only use a pure laparoscopic approach without using transthoracic ports. The study mainly concluded in the feasibility of this approach with an advantage to laparoscopy in terms of complications and hospital stay. This study is really important because it includes a large number of patients but significant technological advances may challenge their conclusions. First of all, during robotic procedures, a port was inserted in the right intercostal space between the 10th and 11th rib. The described RLR technique is thus transthoracic which could perhaps explain the complication rate and difference in hospital stay as they did not use any transthoracic port in the laparoscopic group. Secondly, there is a huge generation gap between the Da Vinci S or Si system used in the Italian report and the Da Vinci X or Xi system, which possesses an articulated energy device and that allows the use of a robotic articulated stapler. In our HPB center, we have carried out some procedures with the Da Vinci S system but it did not convince us about the interest of such system due to several technical limitations. The main limitation was the lack of available energy device for liver parenchymotomy. We now use the Vessel Sealer which combines a sealing device with a bipolar device and is well sized for clamp crushing.
Furthermore, a few meta-analyses comparing robotic to laparoscopic hepatectomy were published recently showing similar feasibility and safety and comparable oncological results [19,20,21]. These studies showed also the feasibility to use robotic resection for major hepatectomy, despite the fact that they were less frequent with only 20% of major robotic resection [21]. The largest meta-analysis was conducted by Kamarajah et al. [21] and included 26 studies and 2630 patients of which 950 had robotic resection. Results of these three studies are similar to ours including no significant differences in blood loss, overall complications, and R0 resection rate. However, we had a significant shorter hospital stay in the RLR group. In two of these meta-analyses [19, 21] operation time was longer in the robotic group which probably results from factors, like set-up and docking time. It is interesting to note that in our study operation time was similar between the two groups. It can be assumed that, despite the set-up and docking time, operative time depends considerably on the surgeon’s experience. In agreement with these studies, Cipriani et al. [22] showed in a multi-centric propensity score-based study that the robotic approach in highly difficult resections represents increased intraoperative safety and could possibly extend the indication of surgery of major hepatectomy.
However, these studies included all type of hepatectomy and did not analyze specifically robotic resections of the PS segments versus the TTL approach. Available data are scarce. Recently, one case series reporting only 5 patients [12] and one retrospective study [23] of the robotic approach of the PS segments were published. Zhao et al. [23] analyzed retrospectively 100 robotic resections of the PS segments of the liver and concluded that this approach was safe and feasible, but without comparing these results to an alternative approach, like laparoscopic resection or TTL.
This is therefore the first report of a comparison between RLR and TTL for PS liver lesions. Yet RLR is an emerging technique for tumors that are difficult to remove by laparoscopy. Furthermore, our experience suggests that RLR may be superior to TTL for two main reasons. The first argument is that postoperative course and intraoperative bleeding are not worse compared to TTL. Pulmonary events and potential of thoracic tumor implantation are clearly less frequent in the RLR group and this is not negligible in the era of enhanced recovery development [23]. We also observed a significantly shorter ICU stay in the RLR group which was consistent with other studies [20, 24]. The second argument lays in the technical possibilities offered by the robotic surgical system like, for example, a stable 3D view and wristed instrumentation with higher grade of mobility and filtration of tremor [25, 26]. The learning curve seems really shortened compared to laparoscopy [27] and our experience shows a significant increase in the difficulty of the interventions performed after a surgical experience of 30 procedures. This appears especially marked regarding procedures in the PS segments. This threshold of 30 RLR has already been reported in the literature [28, 29]. The originality of our work was to demonstrate that, after a really fast learning curve, the robotic approach makes it possible to perform more technically challenging procedures than those that would have been accomplished by traditional laparoscopy. This means that almost half of our RLR would not have been possible in a mini-invasive fashion without the Da Vinci X system. Nota et al. reported in 2019 [13], a multi-centric study comparing RLR to open resections in the PS segments. The hospital length of stay in the RLR group was 4 (3–6) days, which is similar to our study. It was significantly shorter than in the open surgery group (10 days). The switch from open surgery to RLR for 11 patients who would not have been eligible for TTL in our institution theoretically allowed us to save up to 66 days of hospitalization.
Other advantages of the robot can be discussed and may explain why experienced operators prefer this surgical approach. Five ports are sufficient to explore and resect almost all hepatic segments and the interchangeability of the optical port with any other port allows multiple resections with a limited number of wall incisions. In our series, two excluded patients underwent multiple resections (left lateral sectionectomy + wedge resection in segment VII and segmentectomy VI + wedge resection in segment VIII). No additional port was needed to operate on these two cases. In addition, transthoracic accesses are known to cause chronic pain [30]. In our experience, thoracic incision was never necessary during RLR. Ten percent of our RLR were performed for intrahepatic cholangiocarcinoma. This number is even more important in the whole cohort of 118 RLR patients. Indeed, robotic approach is often preferred because of the ease of carrying out the lymphadenectomy of the hepatic pedicle.
However, one major inconvenience of the robotic approach are the economic costs. A recent review [31] showed significantly higher operative, hospitalization, and overall costs for robotic resection versus laparoscopic resection, minor, and major hepatectomy included. Consequently, those costs should be taken into account, but should also be balanced by the above advantages of this innovative approach.
The present study has several limitations. First, there is a selection bias due to the retrospective nature of the study. Secondly, we included a small number of patients which could have led to a lack of statistical power. Randomized trials are needed to lessen these biases and to support our results.
In conclusion, RLR may provide some advantages over TTL for the resection of tumors located in the PS segments and lead to drive more patients to mini-invasive surgery.
References
Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G (2016) Comparative short-term benefits of laparoscopic liver resection: 9000 Cases and Climbing. Ann Surg 263(4):761–777
Couinaud C (1954) Lobes et segments hépatiques: notes sur l’architecture anatomiques et chirurgicale du foie [Liver lobes and segments: notes on the anatomical architecture and surgery of the liver]. Presse Med 62(33):709–712
Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I et al (2009) The international position on laparoscopic liver surgery: the louisville statement, 2008. Ann Surg 250(3):825–830
Soubrane O, Schwarz L, Cauchy F, Perotto LO, Brustia R, Bernard D et al (2015) A conceptual technique for laparoscopic right hepatectomy based on facts and oncologic principles: the caudal approach. Ann Surg juin 261(6):1226–1231
Morise Z (2016) Laparoscopic liver resection for posterosuperior tumors using caudal approach and postural changes: a new technical approach. WJG 22(47):10267
Fuks D, Gayet B (2015) Laparoscopic surgery of postero-lateral segments: a comparison between transthoracic and abdominal approach. Updates Surg juin 67(2):141–145
Inoue Y, Suzuki Y, Fujii K, Kawaguchi N, Ishii M, Masubuchi S et al (2017) Laparoscopic liver resection using the lateral approach from intercostal ports in segments VI, VII, and VIII. J Gastrointest Surg déc 21(12):2135–2143
Ichida H, Ishizawa T, Tanaka M, Terasawa M, Watanabe G, Takeda Y et al (2017) Use of intercostal trocars for laparoscopic resection of subphrenic hepatic tumors. Surg Endosc mars 31(3):1280–1286
Moisan F, Gayet B, Ward MA, Tabchouri N, Fuks D (2018) Segment 7 laparoscopic liver resection: is it possible to resect when metastatic lesions border suprahepatic veins? J Gastrointest Surg sept 22(9):1643–1644
Ocuin LM, Tsung A (2015) Robotic liver resection for malignancy: current status, oncologic outcomes, comparison to laparoscopy, and future applications: current status of robotic hepatectomy for malignancy. J Surg Oncol sept 112(3):295–301
Liu R, Wakabayashi G, Kim HJ, Choi GH, Yiengpruksawan A, Fong Y et al (2019) International consensus statement on robotic hepatectomy surgery in 2018. WJG 25(12):1432–1444
Araujo RLC, Sanctis MA, Barroti LC, Coelho TRV (2020) Robotic approach as a valid strategy to improve the access to posterosuperior hepatic segments—case series and review of literature. J Surg Oncol. https://doi.org/10.1002/jso.25831
Nota CL, Woo Y, Raoof M, Boerner T, Molenaar IQ, Choi GH et al (2019) Robotic versus open minor liver resections of the posterosuperior segments: a multinational, propensity score-matched study. Ann Surg Oncol févr 26(2):583–590
Boleslawski E, Petrovai G, Truant S, Dharancy S, Duhamel A, Salleron J et al (2012) Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. British J Surg 99(6):855–863
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg août 240(2):205–213
Tanaka S, Kubo S, Kanazawa A, Takeda Y, Hirokawa F, Nitta H et al (2017) Validation of a difficulty scoring system for laparoscopic liver resection: a multicenter analysis by the endoscopic liver surgery study group in Japan. J American Coll Surg 225(2):249-258e1
Goodman ZD (2007) Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 47(4):598–607
Montalti R, Scuderi V, Patriti A, Vivarelli M, Troisi RI (2016) Robotic versus laparoscopic resections of posterosuperior segments of the liver: a propensity score-matched comparison. Surg Endosc 30(3):1004–1013
Hu Y, Guo K, Xu J, Xia T, Wang T, Liu N et al (2021) Robotic versus laparoscopic hepatectomy for malignancy: a systematic review and meta-analysis. Asian J Surg 44(4):615–628
Ziogas IA, Giannis D, Esagian SM, Economopoulos KP, Tohme S, Geller DA (2021) Laparoscopic versus robotic major hepatectomy: a systematic review and meta-analysis. Surg Endosc 35(2):524–535
Kamarajah SK, Bundred J, Manas D, Jiao L, Hilal MA, White SA (2021) Robotic versus conventional laparoscopic liver resections: a systematic review and meta-analysis. Scand J Surg 110(3):290–300
Cipriani F, Fiorentini G, Magistri P, Fontani A, Menonna F, Annecchiarico M et al (2021) Pure laparoscopic versus robotic liver resections: multicentric propensity score-based analysis with stratification according to difficulty scores. J Hepatobiliary Pancreat Sci. 23:S731–S732
Zhao Z, Yin Z, Pan L, Li C, Hu M, Lau WY et al (2021) Robotic hepatic resection in postero-superior region of liver. Updates Surg juin 73(3):1007–1014
Fruscione M, Pickens R, Baker EH, Cochran A, Khan A, Ocuin L et al (2019) Robotic-assisted versus laparoscopic major liver resection: analysis of outcomes from a single center. HPB 21(7):906–911
Schmelzle M, Krenzien F, Schöning W, Pratschke J (2021) Möglichkeiten und Grenzen der robotischen Leberchirurgie—aktueller Stand 2020. Chirurg 92(2):107–114
Scognamiglio P, Stüben BO, Heumann A, Li J, Izbicki JR, Perez D et al (2021) Advanced robotic surgery: liver, pancreas, and esophagus—the state of the art? Visc Med 37(6):505–510
Coelho FF (2016) Laparoscopic liver resection: experience based guidelines. WJGS 8(1):5
Zhu P, Liao W, Yang DZ, Chen L, Guang ZW, Xiang ZB et al (2019) Learning curve in robot-assisted laparoscopic liver resection. J Gastrointest Surg 23(9):1778–1787
Efanov M, Alikhanov R, Tsvirkun V, Kazakov I, Melekhina O, Kim P et al (2017) Comparative analysis of learning curve in complex robot-assisted and laparoscopic liver resection. HPB 19(9):818–824
Fiorelli S, Cioffi L, Menna C, Ibrahim M, De Blasi RA, Rendina EA et al (2020) Chronic pain after lung resection: risk factors, neuropathic pain, and quality of life. J Pain Symp Manag 60(2):326–335
Ziogas IA, Evangeliou AP, Mylonas KS, Athanasiadis DI, Cherouveim P, Geller DA et al (2021) Economic analysis of open versus laparoscopic versus robotic hepatectomy: a systematic review and meta-analysis. Eur J Health Econ juin 22(4):585–604
Funding
No funding was received by authors for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
PD, ST, MEA, and GM have no conflicts of interest or financial ties to disclose.
Ethical approval
The study was approved by the Ethics Committee of Lille University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Denglos, P., Truant, S., El Amrani, M. et al. Robotic liver resection in the posterosuperior segments as a way to extent the mini-invasive arsenal: a comparison with transthoracic laparoscopic approach. Surg Endosc 37, 4478–4485 (2023). https://doi.org/10.1007/s00464-023-09919-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-023-09919-6