Abstract
Background
Postoperative pancreatic fistula (POPF) is often associated with significant morbidity and mortality after the Whipple operation. Patient-related factors associated with POPF include soft pancreatic texture and a small main pancreatic duct (MPD). The traditional duct-to-mucosa anastomosis was modified to be easily performed. The aim of the study was to evaluate the simplified pancreaticojejunostomy (PJ) method in the prevention of POPF after minimally invasive pancreaticoduodenectomy (PD).
Methods
Ninety-eight patients who underwent laparoscopic pancreaticoduodenectomy (LPD) and robotic pancreaticoduodenectomy (RPD) with a simplified PJ procedure containing only two duct-to-mucosa sutures and four penetrating-sutures to anastomose the pancreatic parenchyma and jejunal seromuscular layer in our center were retrospectively studied. Demographics and clinical short-term safety were assessed.
Results
All LPD and RPD procedures were successfully performed. The median time of PJ was 17 min, and the median blood loss was 60 mL, with only one patient requiring transfusion. Four patients (4.1%) suffered from clinically relevant POPF (CR-POPF), including four grade B cases and no grade C cases. For patients with an MPD diameter of 3 mm or less, POPF was noted in two (4%) of the fifty patients, with all cases being grade B. Of the patients with a soft pancreas, only two (4.5%) patients suffered from grade B POPF. One patient (1.0%) experienced a 90-day mortality. Neither the main pancreatic diameter nor pancreatic texture had an impact on postoperative outcomes.
Conclusions
Our technique is a simple, safe and efficient alternative to prevent POPF after LPD and RPD. This method is suitable for almost all pancreatic conditions, including cases with a small main pancreatic duct and soft pancreas, and has the potential to become the preferred procedure in low-volume pancreatic surgery centers.
Graphical abstract
Our modified duct-to-mucosa PJ, which contains only two duct-to-mucosa sutures and four penetrating-sutures to anastomose the pancreatic parenchyma and jejunal seromuscular layer, is ideal for small MPD and soft pancreas when performing minimally invasive PD and has a low rate of POPF. PJ pancreaticojejunostomy, MPD main pancreatic diameter, PD pancreaticoduodenectomy, POPF postoperative pancreatic fistula

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pancreaticoduodenectomy (PD) is the standard of therapy for patients with malignant or benign disease of the pancreatic head or periampullary region. In recent years, some doctors have proposed surgical treatment using minimally invasive procedures, including laparoscopic pancreaticoduodenectomy (LPD) and robotic pancreaticoduodenectomy (RPD), which seem to show comparable clinical outcomes, including operative time and R0 resection rate, to open surgery, despite being considered inferior in the past [1,2,3]. Minimally invasive PD also has unique advantages. LPD allows for smaller surgical incisions, and RPD overcomes several limitations related to LPD, such as optical vision, a steep learning curve and surgeon tremor [4,5,6,7]. The transition from open surgery to robotic PD seems to be easier compared with the transition from open surgery to LPD. However, no differences are currently found in clinically relevant parameters between the two minimally invasive approaches [8]. These two minimally invasive procedures have been used in some high-volume pancreatic surgery centers with remarkable results [9].
Postoperative pancreatic fistula (POPF) is often associated with significant morbidity and mortality after the Whipple operation [10,11,12,13,14]. POPF can result in intra-abdominal infection, intra-abdominal hemorrhage, prolonged hospital stays, the need for reoperation or interventional therapy, and even death.
Patient-derived factors associated with pancreatic anastomotic failure have been identified and include soft pancreatic texture, a small MPD and a poor blood supply [15,16,17,18,19]. To reduce the incidence and related complications associated with pancreatic fistula, numerous anastomotic techniques and pharmacologic interventions have been proposed and studied [20,21,22,23,24,25]; however, there is still no accepted standard approach for decreasing pancreatic fistula after PD.
Essential criteria in an “optimal” technique for pancreaticojejunostomy (PJ) should be associated with a low rate of significant pancreatic anastomotic failure-related complications and mortality; additionally, this technique should be easy to learn, perform and duplicate [26]. Two main methods are currently used for PJ anastomosis, including the invagination and “duct-to-mucosa” anastomosic techniques. Pancreatic penetrating-suture has gained wide acceptance in recent years because it is suitable for a soft and fragile pancreas [14, 24, 27, 28]. Penetrating-suture is not only easy to achieve but also reduces the possibility of laceration for a fragile pancreas because more pancreatic parenchyma is bundled by a single suture. In 1996, the Japanese scholar Kakita proposed a new PJ with fewer sutures that used full-thickness sutures to complete anastomosis of the pancreatic stump and jejunal loop for the first time. At the time, this technique had a very low rate of grade B + C POPF (1.2%) [29], and it became the most popular PJ technique in Japan.
Herein, we propose a duct-to-mucosa PJ technique that can be performed in LPD and RPD. The technique was developed based on the Kakita PJ and modified to be simpler and easier. This procedure requires only 6 sutures, and the clinical outcomes were excellent.
Materials and methods
This study was approved by the Medical Ethics Committee of the Zhongnan Hospital of Wuhan University (2022113 K).
Patient selection
From May 2018 to March 2022, 98 consecutive patients underwent minimally invasive PD with modified Kakita PJ in our center. The selection criteria for our study included the following: (1) patients who underwent LPD or RPD and (2) patients who underwent modified Kakita PJ. The exclusion criteria were as follows: (1) patients who underwent OPD; (2) conversion to open surgery; (3) small retrieval incision reconstruction; and (4) other PJ procedures. (Fig. 1).
Clinical information
The demographic characteristics and perioperative details were accurately collected. The diameter of the MPD was measured at the level of the pancreatic neck by computed tomography, and the pancreatic texture was evaluated by the surgeon. For postoperative outcomes, the diagnostic criteria for POPF, biliary fistula, delayed gastric emptying (DGE) and postpancreatectomy hemorrhage (PPH) were classified according to the International Study Group of Pancreatic Surgery (ISGPS) [30]. To verify that our modified Kakita PJ was effective regardless of the pancreatic conditions, we divided patients into two groups according to pancreatic texture and MPD size. Furthermore, we evaluated risk factors for anastomoses for pancreatic surgeries through the four-tier classification system by ISGPS to achieve international comparability [31].
To verify the application of modified Kakita PJ in various preoperative risk groups, we divided patients into low-risk and high-risk groups based on the benchmark case selection criteria to better evaluate the relationship between postoperative complications and preoperative risk in patients who underwent our PJ procedure [32].
Surgeons and pancreatic surgery center
All minimally invasive PDs were performed at the Pancreatic Surgery Center, Zhongnan Hospital of Wuhan University. A total of 100–150 pancreatic surgeries are performed in our center each year, of which 58–80 are PD, and the minimally invasive PD rate is 50–60%. The main surgeon (Dr. Zhiyong Yang) had 8 years of experience with independent PD procedures (nearly 450 OPDs, 42 LPDs and 15 RPDs) prior to the study and performed nearly 90% of minimally invasive PD procedures in our center.
Modified kakita PJ (video)
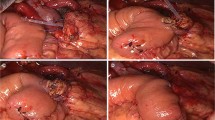
The end of the jejunal loop was closed by a stapler. A small, full-thickness opening was made on the anti-mesenteric border at approximately 6 cm to the jejunal stump. A 1–3 mm stent was inserted into the MPD remnant (Fig. 2).
To anastomose the pancreatic stump and jejunal wall, needles with a 3–0 non-absorbable Prolene (Ethicon, 8842, 36 mm, 1/2c) suture were used in an end-to-side penetrating pattern. The first stitch (15 cm in length), which was close to the MPD, completely penetrated the pancreatic parenchyma from anterior to posterior and then from posterior to anterior, traversing the seromuscular layer of the jejunum wall. The edge distance was approximately 1.0 cm on the pancreatic stump, while the stitch width on the jejunum wall was slightly larger than the pancreatic stump thickness. When finished, the pancreatic stump and jejunal wall were drawn close (Fig. 3).
Then, to anastomose the MPD and jejunal mucosa, needles with a 4–0 absorbable Vicryl (Ethicon, VCP771D, 22 mm, 1/2c) suture were used. The second stitch (12 cm in length) penetrated the pancreas from the duct to the posterior stump and entered the jejunum wall posterior to the jejunum hole. The stitch of the MPD’s posterior wall was knotted prior to stent insertion into the jejunum (Fig. 4).
At this point, the second penetrating stitch (12 cm in length) to anastomose the pancreatic stump and jejunal wall was completed and knotted. This stitch was opposite to the first stitch and was also close to the MPD (Fig. 5).
Next, to suture the anterior wall of the MPD and jejunal mucosa, the fourth stitch (12 cm in length) was knotted with a 4–0 absorbable Vicryl suture. Subsequently, the first penetrating stitch was knotted (Fig. 6).
Finally, the last two sutures (12 cm in length), which were used to anastomose the upper and lower borders of the pancreatic stump and jejunal wall, were knotted to compete the anastomosis (Fig. 7).
Further reconstruction for digestive continuity was completed by using the Child procedure, including choledochojejunostomy and gastrojejunostomy. Then, three drainage tubes were placed posterior to the choledochojejunostomy and superior and posterior to the pancreaticojejunostomy.
Postoperative management
Sandostatin was routinely used after surgery to prevent possible POPF. The amylase concentration of drainage was measured on postoperative days (PODs) 3 and 5, as well as at subsequent time points as necessary. Computed tomography scans (with contrast enhancement) were obtained to assess the fluid collection and guide the management of the drains for each patient on PODs 5–7. For patients with a low-risk of POPF and less than 3000 U/L of amylase, the drainage tube was retreated on POD 3 and then removed on PODs 5–7; for patients with a high-risk of POPF, the time of drainage tube placement needed to be appropriately extended; for patients with POPF, the drains were removed when the total output was < 10 mL for three consecutive days unless fever or infection occurred.
Statistical analysis
Values are expressed as percentages and medians, according to their distributions. The Mann‒Whitney U test was used for non-normally distributed variables, and the chi-square test or Fisher’s exact test was applied to the categorical data. All statistical analyses were performed using SPSS version 25.0 software (SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
Results
The patients in our study included 57 males and 41 females with a median age of 63 years old (11–77 years old). The median BMI of the patients was 21.43 kg/m2 (15.92–32.44 kg/m2). Sixty-six patients (66.3%) were diagnosed with jaundice (Table 1).
For the intraoperative situation, the median duration of PD was 350 min (260–480 min), and the median time for performing PJ was 17 min (12–25 min). The median blood loss was 60 mL (10–250 mL), and one patient (1.0%, 1/98) who suffered moderate anemia before the operation received a blood transfusion (Table 2). The pathologic results are listed in Table 3.
POPF, based on the international study group on pancreatic fistula definition in 2017 [30], occurred in 4 patients (4.1%). These fistulas were all grade B and were treated successfully by prolonging the time of drainage and percutaneous drain placement (1 patient). Grade A POPFs, which is no longer considered a CR-POPF, were designated as “biochemical leaks” and occurred in 18 patients (18.4%). The median amylase level in the drainage fluid on POD 3 was 87 U/L in all patients. Three patients (3.1%) suffered from an intra-abdominal hemorrhage, and one of them had a right hepatic artery hemorrhage, which was cured by interventional therapy. Another two patients required reoperations; one of them had hemorrhage due to perforation of the jejunal stump, which may have been punctured by the pancreatic duct stenting tube on postoperative day (POD) 24, and the other patient had hemorrhage from the stomach stump on POD 27 due to its perforation. Biliary fistula occurred in 5 patients (5.1%) whose common bile ducts were not dilated. These patients were cured by prolonging the time of drainage and percutaneous drain placement (2 patients). In addition, there were 6 patients (6.1%) with delayed gastric emptying, which resolved after conservative treatment, such as parenteral or enteral nutrition. There were 8 patients (8.2%) with intra-abdominal infection. Of these, three patients were cured by antibiotic therapy with prolonged drainage, and four patients were treated successfully by percutaneous drain placement. One patient died on POD 23 because of severe septic shock that developed from afferent loop obstruction and perforation. Three patients (3.1%) with pulmonary infection were cured by antibiotic therapy. There were no cases of urinary tract infection, and no patients died as a result of POPF. The median postoperative hospital stay was 17 days (9–101 days) (Table 4). It should be stated that Chinese patients usually do not want to be discharged unless their abdominal drains are removed. Therefore, the postoperative hospital stay of our patients was much longer than that in other reports.
After grouping according to the diameter of the MPD and the texture of the pancreas, we found that there were no significant differences (P > 0.05) in patient characteristics, pathological outcomes, or perioperative situation, including POPF (Tables 5–6). The rates of CR-POPF did not differ significantly (P < 0.05) among the four grades through the four-tier classification system according to the ISGPS. The occurrence rates of grade A–D were 3.7%, 3.7%, 4.8%, and 4.4%, respectively (Table 7).
Based on the selection criteria of the benchmark cases, 27 (27.6%) high-risk (benchmark) and 71 (72.4%) low-risk (non-benchmark) patients constituted the cohort in our study (Table 8). When comparing the postoperative complications between the two groups, the incidence of CR-POPF and intra-abdominal infection in the high-risk group was seemingly higher than that in the low-risk group, but the differences were not statistically significant. The occurrence rates of other complications, such as biliary fistula, DGE, and intra-abdominal hemorrhage, were similar in both groups.
Discussion
For Kakita PJ anastomosis [24], several sutures should be performed within the MPD, which is rather challenging when the duct is not dilated. According to recent studies, for patients with a small MPD, grade B + C POPF was observed in 6.7% to 19.2% [14, 33, 34], which was troubling to many surgeons.
Initially, we anastomosed the duct to the mucosa by using an interrupted circular suture containing 6 to 8 stitches in OPD. This was theoretically difficult to perform in LPD and RPD due to the narrow operating field view and was prone to cause pancreatic parenchyma lacerations when knotted. The critical change made was to gradually reduce the duct-to-mucosa stitch number from 6 to 8 stitches to 4 stitches, after which anastomosis remained reliable. Finally, we found that just 2 stitches anterior and posterior to the duct were sufficient in OPD. Additional stitches were needed only in the case of an extremely dilating duct. In this series, the maximum MPD was 6 mm, which was also applied to our PJ procedure.
The grade B + C POPF rate (4.1%) was extremely low and much lower than that currently reported in other studies. All POPF cases were cured by simple drainage or abdominal paracentesis. To this end, we analyzed the clinical data of OPDs performed with different PJ techniques in our center from January 2014 to April 2018 and found that the grade B + C POPF rate was 13.5% (11.7% grade B, 1.8% grade C), which was higher than that of our PJ. In summary, reducing the stitch number not only simplified the anastomotic procedure and decreased the difficulty of the operation, especially in LPD and RPD, but also enabled a “duct-to-mucosa” PJ with a penetrating-suture to better suit small-bore MPD patients. To our knowledge, the 2-stitch technique anastomosing the duct and mucosa was much simpler than that used by other surgeons who used penetrating-suture in PD [20, 22, 24].
To further validate the applicability of modified Kakita PJ in anastomoses with different risks, all patients were grouped according to different pancreatic textures and MPD diameters and the four-tier classification system proposed by ISGPS for international comparability [31]. These grouping results showed that, even in cases with a soft pancreas and small MPD, the incidence of CR-POPF was only 4.4%, which indicated that our PJ was effective for cases with different pancreatic textures and MPD diameters.
In addition to the inherent factors of the pancreas, the anastomotic technique, especially the maintenance of blood supply to the PJ, also had a crucial impact on the incidence of POPF. In the conventional duct-to-mucosa or invagination PJ, the pancreatic remnant is mobilized with approximately 0.5–1 cm reserved for the posterior layer suture. This method is not only laborious in some patients, such as in those with chronic pancreatitis, but may also harm the blood supply. Dissociation of the pancreatic remnant was not mandatory in our method, which allowed the needles to puncture from the posterior edge of the pancreatic stump. In addition to protecting the blood supply of the stump, this anastomosis technique is seemingly simplified. This additional unique advantage of our approach may contribute to the reduction in the occurrence of POPF.
For minimally invasive PD, the long learning curve is often difficult for surgeons to overcome. A systematic review showed that 39 cases of LPD and 25 cases of RPD could be recognized as the first stage of the learning curve [35]. According to the cases of minimally invasive PD (42 LPDs and 15 RPDs) performed by the main surgeon before the study combined with the OPD experience of 6 years before the LPD procedure, we thought we had completed the first phase of the learning curve, and the learning period of RPD could be classified as the terminal of phase I. An increasing number of studies have proven that there would be more complex cases in the period of technical competence and challenge [35,36,37], which could be demonstrated in our study’s pathological results of the high proportion of malignant tumors. However, our study did not report a case of minimally invasive PD combined with blood vessel reconstruction, which was also the goal we would challenge in the future. As for digestive tract reconstruction, PJ tends to be the most difficult step. Simplicity and a short learning curve are required for the optimal PJ technique. Our PJ was performed in OPD at first. After 52 cases of OPD were completed, we started to perform PJ under laparoscopy and then robotically. Although performing PJ under laparoscopy is challenging, we achieved stabilization of the PJ procedure in fewer than 20 cases. Unlike in the three-phase model of the PD learning curve [35,36,37], the “challenging period” that existed in PD was not suitable for our PJ because once the PJ procedure was established, it could be performed easily in all cases.
Given that some low-volume pancreatic surgery centers do not have much access to cases suitable for minimally invasive PD, it is difficult for them to learn complex PJ procedures. Our PJ, which is simple, safe and effective, has the potential to become the preferred procedure in low-volume pancreatic surgery centers.
However, subject to retrospective study, there were many limitations in our study, including small sample size, single center study and inherent selection bias. The majority of cases (72.4%) were low-risk in our study, and the incidence of CR-POPF in high-risk cases was 11.1%, which indicates that more clinical data on performing PJ in high-risk cases are needed. To further confirm the reliability of this PJ technique, it is necessary to conduct relevant randomized controlled trials in the near future.
Conclusion
To our knowledge, this modified Kakita PJ procedure is the easiest method to perform using LPD and RPD. This technique is suitable for almost all pancreatic conditions, even in cases with a fragile pancreas stump or small MPD, and it has the potential to become the preferred laparoscopic or robotic PJ procedure in low-volume pancreatic surgery centers.
References
van Oosten AF, Ding D, Habib JR, Irfan A, Schmocker RK, Sereni E, Kinny-Koster B, Wright M, Groot VP, Molenaar IQ, Cameron JL, Makary M, Burkhart RA, Burns WR, Wolfgang CL, He J (2021) Perioperative outcomes of robotic pancreaticoduodenectomy: a propensity-matched analysis to open and laparoscopic pancreaticoduodenectomy. J Gastrointest Surg 25:1795–1804
Gall TM, Pencavel TD, Cunningham D, Nicol D, Jiao LR (2020) Transition from open and laparoscopic to robotic pancreaticoduodenectomy in a UK tertiary referral hepatobiliary and pancreatic centre - early experience of robotic pancreaticoduodenectomy. HPB (Oxford) 22:1637–1644
Kamarajah SK, Bundred J, Marc OS, Jiao LR, Manas D, Abu Hilal M, White SA (2020) Robotic versus conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol 46:6–14
Giulianotti PC, Mangano A, Bustos RE, Gheza F, Fernandes E, Masrur MA, Gangemi A, Bianco FM (2018) Operative technique in robotic pancreaticoduodenectomy (RPD) at University of Illinois at Chicago (UIC): 17 steps standardized technique : lessons learned since the first worldwide RPD performed in the year 2001. Surg Endosc 32:4329–4336
Nassour I, Choti MA, Porembka MR, Yopp AC, Wang SC, Polanco PM (2018) Robotic-assisted versus laparoscopic pancreaticoduodenectomy: oncological outcomes. Surg Endosc 32:2907–2913
Liu R, Zhang T, Zhao ZM, Tan XL, Zhao GD, Zhang X, Xu Y (2017) The surgical outcomes of robot-assisted laparoscopic pancreaticoduodenectomy versus laparoscopic pancreaticoduodenectomy for periampullary neoplasms: a comparative study of a single center. Surg Endosc 31:2380–2386
Liao CH, Wu YT, Liu YY, Wang SY, Kang SC, Yeh CN, Yeh TS (2016) Systemic review of the feasibility and advantage of minimally invasive pancreaticoduodenectomy. World J Surg 40:1218–1225
Müller PCM-SB, Hackert T, Nickel F (2022) Robotic pancreaticoduodenectomy after the learning curve—a new hope. Hepatobiliary Surg Nutr 11:489–491
Ouyang L, Zhang J, Feng Q, Zhang Z, Ma H, Zhang G (2022) Robotic versus laparoscopic pancreaticoduodenectomy: an up-to-date system review and meta-analysis. Front Oncol 12:834382
McMillan MT, Christein JD, Callery MP, Behrman SW, Drebin JA, Hollis RH, Kent TS, Miller BC, Sprys MH, Watkins AA, Strasberg SM, Vollmer CM Jr (2016) Comparing the burden of pancreatic fistulas after pancreatoduodenectomy and distal pancreatectomy. Surgery 159:1013–1022
Zhou Y-MZX-F, Wu L-P, Su X, Li B, Shi L-H (2014) Pancreatic fistula after central pancreatectomy: case series and review of the literature. Hepatobiliary Pancreat Dis Int 13:203–208
Yekebas EF, Wolfram L, Cataldegirmen G, Habermann CR, Bogoevski D, Koenig AM, Kaifi J, Schurr PG, Bubenheim M, Nolte-Ernsting C, Adam G, Izbicki JR (2007) Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg 246:269–280
Fuks D, Piessen G, Huet E, Tavernier M, Zerbib P, Michot F, Scotte M, Triboulet JP, Mariette C, Chiche L, Salame E, Segol P, Pruvot FR, Mauvais F, Roman H, Verhaeghe P, Regimbeau JM (2009) Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg 197:702–709
Liu Q, Zhao Z, Gao Y, Zhao G, Tan X, Wang C, Liu R (2020) Novel single-layer continuous suture of pancreaticojejunostomy for robotic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 27:56–63
Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, Mantovani W, Pederzoli P (2005) Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg 242:767–771 (discussion 771-763)
Xu J, Zhang B, Shi S, Qin Y, Ji S, Xu W, Liu J, Liu L, Liu C, Long J, Ni Q, Yu X (2015) Papillary-like main pancreatic duct invaginated pancreaticojejunostomy versus duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Surgery 158:1211–1218
Cai H, Lu F, Zhang M, Cai Y, Wang X, Li Y, Meng L, Gao P, Peng B (2022) Pancreaticojejunostomy without pancreatic duct stent after laparoscopic pancreatoduodenectomy: preliminary outcomes from a prospective randomized controlled trial. Surg Endosc 36:3629–3636
Tanaka K, Yamada S, Sonohara F, Takami H, Hayashi M, Kanda M, Kobayashi D, Tanaka C, Nakayama G, Koike M, Fujiwara M, Kodera Y (2021) Pancreatic fat and body composition measurements by computed tomography are associated with pancreatic fistula after pancreatectomy. Ann Surg Oncol 28:530–538
Zhou Y, Yang J, Wei L, Lin Q, Zheng S, Liu G, Zhou Q, Tan X, Chen R (2021) A novel anastomosis technique facilitates pancreaticojejunostomy in total laparoscopic pancreaticoduodenectomy (with video). Langenbecks Arch Surg 406:2891–2897
Chen XP, Huang ZY, Lau JW, Zhang BX, Zhang ZW, Chen YF, Zhang WG, Zhu P, Zhang B (2014) Chen’s U-suture technique for end-to-end invaginated pancreaticojejunostomy following pancreaticoduodenectomy. Ann Surg Oncol 21:4336–4341
Liu CZ, Zhu JK, Xu Q, Liu FY, Wang YD, Zhu M (2018) Application of pancreaticojejunostomy with one-layer suture in pancreaticoduodenectomy: a retrospective cohort study. Int J Surg 56:68–72
Zhang L, Li Z, Wu X, Li Y, Zeng Z (2015) Sealing pancreaticojejunostomy in combination with duct parenchyma to mucosa seromuscular one-layer anastomosis: a novel technique to prevent pancreatic fistula after pancreaticoduodenectomy. J Am Coll Surg 220:e71-77
Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN (2010) Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg 210:54–59
Kakita ATT, Yoshida M, Furuta K (1996) A simpler and more reliable technique of pancreatojejunal anastomosis. Surg Today 26:532–535
Sultania M, Garg PK, Rajan DK, Pandey D (2022) Pancreaticogastrostomy: a novel technique with duct-parenchyma-gastric wall anastomosis. J Surg Oncol 125:179–184
Olakowski M, Grudzinska E, Mrowiec S (2020) Pancreaticojejunostomy-a review of modern techniques. Langenbecks Arch Surg 405:13–22
Chen Y, Zhu X, Huang J, Zhu Y (2015) End-to-side penetrating-suture pancreaticojejunostomy: a novel anastomosis technique. J Am Coll Surg 221:e81-86
Fujii T, Sugimoto H, Yamada S, Kanda M, Suenaga M, Takami H, Hattori M, Inokawa Y, Nomoto S, Fujiwara M, Kodera Y (2014) Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg 18:1108–1115
Kakita A, Yoshida M, Takahashi T (2001) History of pancreaticojejunostomy in pancreaticoduodenectomy: development of a more reliable anastomosis technique. J Hepatobiliary Pancreat Surg 8:230–237
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M, International Study Group on Pancreatic S (2017) The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 161:584–591
Schuh F, Mihaljevic AL, Probst P, Trudeau MT, Müller PC, Marchegiani G, Besselink MG, Uzunoglu F, Izbicki JR, Falconi M, Fernandez-Del Castillo C, Adham M, Z’graggen K, Friess H, Werner J, Weitz J, Strobel O, Hackert T, Radenkovic D, Kelemen D, Werner J, Weitz J, Strobel O, Hackert T, Radenkovic D, Kelemen D, Wolfgang C, Miao YI, Shrikhande SV, Lillemoe KD, Dervenis C, Bassi C, Neoptolemos JP, Diener MK, Vollmer CM Jr, Büchler MW (2021) A simple classification of pancreatic duct size and texture predicts postoperative pancreatic fistula: a classification of the international study group of pancreatic surgery (ISGPS). Ann Surg. https://doi.org/10.1097/SLA.0000000000004855
Sanchez-Velazquez P, Muller X, Malleo G, Park JS, Hwang HK, Napoli N, Javed AA, Inoue Y, Beghdadi N, Kalisvaart M, Vigia E, Walsh CD, Lovasik B, Busquets J, Scandavini C, Robin F, Yoshitomi H, Mackay TM, Busch OR, Hartog H, Heinrich S, Gleisner A, Perinel J, Passeri M, Lluis N, Raptis DA, Tschuor C, Oberkofler CE, DeOliveira ML, Petrowsky H, Martinie J, Asbun H, Adham M, Schulick R, Lang H, Koerkamp BG, Besselink MG, Han HS, Miyazaki M, Ferrone CR, Fernandez-Del Castillo C, Lillemoe KD, Sulpice L, Boudjema K, del Chiaro M, Fabregat J, Kooby DA, Allen P, Lavu H, Yeo CJ, Barroso E, Roberts K, Muiesan P, Sauvanet A, Saiura A, Wolfgang CL, Cameron JL, Boggi U, Yoon DS, Bassi C, Puhan MA, Clavien PA (2019) Benchmarks in pancreatic surgery: a novel tool for unbiased outcome comparisons. Ann Surg 270:211–218
Chen H, Wang W, Ying X, Deng X, Peng C, Cheng D, Shen B (2020) Predictive factors for postoperative pancreatitis after pancreaticoduodenectomy: a single-center retrospective analysis of 1465 patients. Pancreatology 20:211–216
Ma D, Du G, Yang J, Song J, Ma H, Wang J, Zhang T, Jin B (2021) Clinical application of a modified double purse-string continuous suture technique for pancreaticojejunostomy: reliable for laparoscopic surgery and small size main pancreatic duct. Biomed Res Int 2021:6676999
Müller PC, Kuemmerli C, Cizmic A, Sinz S, Probst P, de Santibanes M, Shrikhande SV, Tschuor C, Loos M, Mehrabi A, Z’graggen K, Müller-Stich BP, Hackert T, Büchler MW, Nickel F (2022) Learning curves in open, laparoscopic, and robotic pancreatic surgery. Ann Surg Open 3:e111
Wang M, Meng L, Cai Y, Li Y, Wang X, Zhang Z, Peng B (2016) Learning curve for laparoscopic pancreaticoduodenectomy: a CUSUM analysis. J Gastrointest Surg 20:924–935
Choi M, Hwang HK, Lee WJ, Kang CM (2021) Total laparoscopic pancreaticoduodenectomy in patients with periampullary tumors: a learning curve analysis. Surg Endosc 35:2636–2644
Acknowledgements
The manuscript was written by all the authors. All authors have given approval for the final version of the manuscript.
Funding
This work was supported by the Grants from Clinical Medicine Research Center for Minimally Invasive Procedure of Hepatobiliary & Pancreatic Diseases of Hubei Province (PTYX2022009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Anbang Zhao, Qian Zhu, Xian Qin, Kunlei Wang, Kai Tan, Zhicheng Liu, Wenjing Song, Qian Cheng, Xinyin Li, Zhinan Chen, Zhisu Liu, Yufeng Yuan and Zhiyong Yang have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 307902 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, A., Zhu, Q., Qin, X. et al. A duct-to-mucosa pancreaticojejunostomy for small main pancreatic duct and soft pancreas in minimally invasive pancreaticoduodenectomy. Surg Endosc 37, 3567–3579 (2023). https://doi.org/10.1007/s00464-022-09830-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-022-09830-6