Abstract
Introduction
Non-healing of anastomotic leakage can be observed in up to 50% after total mesorectal excision for rectal cancer. This study investigates the efficacy of early transanal closure of anastomotic leakage after pre-treatment with the Endosponge® therapy.
Methods
In this prospective, multicentre, feasibility study, transanal suturing of the anastomotic defect was performed after vacuum-assisted cleaning of the presacral cavity. Primary outcome was the proportion of patients with a healed anastomosis at 6 months after transanal closure. Secondary, healing at last follow-up, continuity, direct medical costs, functionality and quality of life were analysed.
Results
Between July 2013 and July 2015, 30 rectal cancer patients with a leaking low colorectal anastomosis were included, of whom 22 underwent neoadjuvant radiotherapy. Median follow-up was 14 (7–29) months. At 6 months, the anastomosis had healed in 16 (53%) patients. At last follow-up, anastomotic integrity was found in 21 (70%) and continuity was restored in 20 (67%) patients. Non-healing at 12 months was observed in 10/29 (34%) patients overall, and in 3/14 (21%) when therapy started within three weeks following the index operation. Major LARS was reported in 12/15 (80%) patients. The direct medical costs were €8933 (95% CI 7268–10,707) per patient.
Conclusion
Vacuum-assisted early transanal closure of a leaking anastomosis after total mesorectal excision with 73% preoperative radiotherapy showed that acceptable anastomotic healing rates and stoma reversal rates can be achieved. Early diagnosis and start of treatment seems crucial.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Anastomotic leakage is still one of the most dreaded complications following rectal cancer surgery using total mesorectal excision (TME) [1]. Extensive research has focussed on predisposing factors. The common thought is that the leak is being caused by a broad spectrum of both adjustable and non-adjustable factors [2]. Despite optimising surgical techniques (minimal invasive surgery) and perioperative management (ERAS), the leakage rates of colorectal anastomosis remained high (8–20%) over time [2,3,4,5,6,7,8,9]. The TME creates a presacral cavity behind the anastomosis where large amounts of debris and pus can accumulate even in case of a minor anastomotic dehiscence. The anal sphincter functions as a physiologic barrier preventing drainage of the abscess cavity and neorectum via the anus.
Traditionally, if a patient presents with a symptomatic leak, the anastomosis is defunctioned, if not done so primarily. The abscess is most commonly drained either percutaneously or transanally. Half of these leaks might heal with this conventional management [10]. But if these leaks do not heal, a chronic presacral sinus is formed. The sinus might be asymptomatic, but can also be a source of major morbidity even up to life-threatening necrotising fasciitis of the upper leg [11]. Management of the chronic sinus means major surgery taking down the leaking anastomosis followed by either redo anastomosis or intersphincteric proctectomy with omentoplasty and permanent colostomy [12]. The Dutch TME trial showed that after secondary stoma formation for infectious problems, the deviating stoma could not be reversed in 49% with preoperative radiotherapy as an independent predictor (HR 0.34) [13].
Since a chronic sinus requires extensive surgical intervention, it is of great importance to prevent the leak to become a chronic sinus. A minimal invasive treatment strategy with vacuum-assisted drainage (EVAC) combined with early transanal closure of the anastomotic defect (‘vacuum-assisted early transanal closure’) has been very successful in the early management of leaking ileoanal anastomoses for ulcerative colitis (UC) or familial polyposis (FAP) [14]. However, these patients have a neorectum made of small bowel instead of colon, and did not receive neoadjuvant radiotherapy. For this reason we aimed to study the efficacy of vacuum-assisted early transanal closure for rectal cancer patients in terms of anastomotic healing, stoma closure, functionality of the neorectum, quality of life (QoL) and treatment-related costs.
Methods
This prospective, multicentre, feasibility study, was carried out in a total of five hospitals throughout the Netherlands that performed vacuum-assisted early transanal closure. Between July 2013 and July 2015, eligible patients with an anastomotic leak after TME surgery from the study centre or a referring hospital were included. Patients who underwent TME for rectal cancer with primary anastomosis up to 6 cm from the anal verge and a confirmed leak by either CT-scan or sigmoidoscopy were considered eligible. Prior to start of EVAC, the anastomosis needed to be defunctioned, if not done so at the index operation. During the exchanges of the Endosponge® (B. Braun Medical B.V., Melsungen, Germany), the final decision was made whether closure of the anastomosis was feasible. Closure was considered feasible if the anastomotic edges at the level of the dehiscence were approximating during desufflation with the endoscope. This could be considered as a sign of sufficient flexibility of both sides of the bowel wall to bring the edges together.
Patients that were considered not suitable for closure of the anastomotic defect could proceed with EVAC, thereby gradually tapering the size of the Endosponge®. Such patients eventually being treated according to the initial description of EVAC without transanal suturing were excluded from the present study [15]. The Institutional Review Board of the Academic Medical Centre in Amsterdam granted exemption from Ethics approval for this study; however, informed consent was requested for sending out the functionality and quality of life questionnaires.
Vacuum-assisted early transanal closure
Our group described the method of vacuum-assisted early transanal closure for patients with a leaking ileal pouch-anal anastomosis (IPAA) in a previous communication [14, 16]. We refer to those papers for extensive description of the technique [14, 16]. Briefly, the Endosponges® were placed endoscopically under light sedation (dormicum/fentanyl). One or two open-pored polyurethane Endosponges® were placed via a plastic tube under the guidance of the endoscope into the deepest point of the abscess cavity. During subsequent placements, the Endosponges® were not tapered, because the objective of the EVAC therapy was to clean the cavity prior to closure of the anastomotic dehiscence with induction of granulation tissue. This is different from EVAC therapy aiming at closure, where the Endosponges® were gradually tapered in order to achieve a collapse of the cavity [17]. The Endosponges® are connected to a low-vacuum suction bottle (Redyrob® TRANS PLUS suction device, Melsungen, Germany) and changed every 3–4 days to prevent tissue ingrowth. When the abscess cavity was considered clean (i.e. granulation tissue covering the abscess cavity) and bowel edges were expected to come together, the anastomotic defect was closed surgically. Closure of the leak was performed under general anaesthesia with the help of a Lone Star Retractor® (Cooper Surgical, Trumbull, United Stated) or via transanal minimal invasive surgery (TAMIS) using the GelPOINT ® Path Transanal Access Platform (Applied Medical, Rancho Santa Margarita, United States) depending on the distance of the anastomosis from the anal verge. Suturing was done using 2-0 Novosin interrupted sutures (B. Braun Medical B.V., Melsungen, Germany). A drain was placed transanastomotic or perianastomotic in the cavity and removed on the third or fourth postoperative day. Patients were treated with antibiotics up to the tenth postoperative day. The reconstructed anastomosis was evaluated by endoscopic inspection and subsequent contrast imaging studies, two weeks after the transanal closure. If no healed anastomosis was observed at this follow-up visit, either a ‘wait and see’ strategy was chosen or a secondary period of EVAC was commenced, or a second attempt to close the defect transanally, based on the size of the remaining leak. The treatment strategy flow chart is presented in Fig. 1. A member of the steering committee attended at least one transanal closure procedure in the other participating centres. No proctoring courses were organised.
Definitions
An anastomosis was considered to be healed if there were no signs of contrast extravasation during abdominal CT or contrast enema and there was an intact anastomosis during endoscopy. If patients required redo surgery with excision of the anastomosis with either end colostomy or a new coloanal anastomosis, they were considered as treatment failures. Stoma closure was considered successful if no subsequent pelvic abscess developed during follow-up, if there was no need of recreation of the stoma and if no other complications had occurred related to anastomotic failure. A chronic sinus was defined as a presacral abscess that was still present 12 months onwards after the index operation.
Outcomes
The primary endpoint was the number of healed anastomoses after 6 months following the first attempt to close the leak surgically. Secondary outcomes were the number of healed anastomoses at the end of follow-up, the number of chronic sinuses, number of patients with successfully restored continuity, quality of life, functionality and direct medical costs. A subanalysis was made between patients that started with EVAC within three weeks (early EVAC group) from the index operation and those where the EVAC started outside this period (late EVAC group), hypothesising that vacuum-assisted early transanal closure is more effective when started early.
Quality of life and functionality analysis
Quality of life (QoL) and function were assessed at fixed time-points after the index operation. QoL was assessed using the Short Form 36 (SF-36®), the GIQLI (Gastrointestinal Quality of Life Index) questionnaire and the EQ-5D-5L at 3, 6, 9 and 12 months postoperative [18,19,20]. Function was assessed in patients where the continuity was restored, using the Colorectal Functional Outcome (COREFO) scale at 6, 9 and 12 months post construction of the anastomosis and the low anterior resection syndrome (LARS) score at the end of the follow-up period [21, 22].
Cost analysis
The direct medical costs that were related to vacuum-assisted early transanal closure were calculated from first EVAC to a healed anastomosis. In the case no anastomotic healing occurred, the cost from first EVAC to the decision to definitively refrain from the minimally invasive strategy was calculated (i.e. decision to perform a redo procedure, to construct an end colostomy or decision to definitively not reverse the stoma, also see red dotted line of Fig. 3). Failed patients either had a redo anastomotic pull-through procedure, an intersphincteric resection with permanent colostomy or the intentionally temporary defunctioning ileostomy became permanent. The costs associated with stoma reversal, the redo procedure itself or subsequent complications are not directly associated with vacuum-assisted early closure and are therefore presented in the Appendix 2. Units that were included in the cost analysis were number of EVACs performed, length of stay following transanal closure, readmissions, interventions, total length of hospital days during follow-up, endoscopic examinations, radiological imaging, outpatient clinic visits and emergency room visits. Unit costing was based on the Dutch costing manual for healthcare research. Costs related specifically to the EVAC or stoma-related costs were determined based on earlier cost-analyses from our study group [14, 23]. The unit costs were determined for the year 2015, after price-indexing (based on general consumer price indices; www.cvz.nl, access date 12 October 2016) of unit costs stemming from different calendar years.
Statistical analysis
According to their distribution, descriptive data are reported as median with range or mean with a standard deviation (SD). As the sample size of this feasibility study was small, no formal comparisons of subgroups were performed. Therefore, no Chi-squared or Fischer’s exact tests were used. In order to analyse the time to healed anastomosis and time to continuity, the Kaplan–Meier method was used. In order to analyse the QoL questionnaires over time, the Wilcoxon-signed rank test was used. All analyses were performed with IBM SPSS statistics, version 23.00 (IBM Corp Armonk, NY, United States).
Results
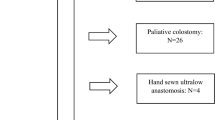
Between July 2013 and July 2015, a total of 45 patients were counselled for vacuum-assisted early closure. A third of these patients (n = 15) were considered not eligible. The patient flow chart and reasons for exclusion are presented in Fig. 2. Finally, a total of 30 patients were included for analysis of the primary endpoint being a healed anastomosis at 6 months from transanal closure.
Patient characteristics
All patients had prior TME surgery for rectal cancer, of whom 27 patients had a primary leaking anastomosis and three patients had undergone a redo coloanal anastomosis with recurrent leakage. The majority of patients were male, and the median age was 66 (40–79) years. Seven patients were not initially diverted and received a diverting ileostomy after the leak was diagnosed. Any form of neoadjuvant therapy was applied in 22 (73%) patients. Median follow-up was 14 (7–29) months.
The baseline characteristics are presented in Table 1.
The leak was located posteriorly in the rectum in 22 patients and a near complete dehiscence of the anastomosis was found in two patients. Median time from the index operation to diagnosis of the leak was 14 (3–75) days. Time from surgery to first Endosponge® placement was median 23 (3–158) days, and EVAC started within three weeks from the index operation in 15 patients. In 9 patients, the defect of the anastomosis needed to be dilated with a 12 mm endoscopic CRE balloon in order to enter the abscess cavity. Transanal closure was performed after a median of 13 (5–51) days and a median of four (2–15) placements. No adverse events related to the EVAC procedures were reported. The details on leakages, EVAC procedures and transanal closures is presented in Table 2.
Considering the transanal closures, median operation time was 70 (26–257) min. The defect was closed with the use of a GelPOINT path in 13 (43%) and with a Lone Star Retractor® in 17 (67%) patients. No adverse events related to the transanal closure occurred. The perianastomotic drain was removed after a median of four (2–6) days in the outpatient clinic. Length of hospital stay following the transanal closure was a median of 1.5 (0–21) days.
Primary endpoint
In 16/30 patients (53%) the anastomosis was healed within 6 months after transanal closure. If EVAC was started within three weeks after the index operation (early EVAC group), anastomotic healing at 6 months was observed in 10/15 (67%) patients, compared to 6/15 (40%) patients with start of EVAC beyond three weeks (late EVAC group). In patients that did not have neoadjuvant therapy, the anastomosis was healed within 6 months in 7 out of 8 patients. Median time for the anastomosis to heal was 127 (14–722) days. At the end of follow-up, the anastomosis had healed in 21/30 (70%) patients. Corresponding rates for the early and late EVAC subgroups were 11/15 (73%) and 10/15 (67%), respectively. The anastomotic leak developed into a chronic sinus in 10/29 (34%) of the patients. In the early EVAC group, 3/14 (21%) patients developed a chronic sinus, compared to 7/15 (47%) in the late EVAC group (Table 3). Interestingly, in three of the patients with a chronic sinus, the anastomosis healed at the end of follow-up.
Figure 3 presents the strategy applied when no healed anastomosis was observed during sigmoidoscopy at two weeks following the transanal closure. Twenty-eight patients had a persistent leak (93%) at 2 weeks follow-up. In one of these patients, it was decided to take-down the anastomosis because of dehiscence that was larger than 270 degrees. Of the remaining 27 patients, 12 were treated conservatively with monthly sigmoidoscopy follow-up. This strategy was chosen if the remaining dehiscence was too small to restart Endosponge® therapy. In 12 patients, the defect was larger and a second Endosponge® therapy was started. This was followed by a second attempt of transanal closure of the defect in 3 of these 12 patients. In two patients, the persisting defect was considered clean enough, and it was decided to directly attempt a second transanal closure, without pre-treatment with Endosponge®. The remaining patient had a presacral abscess without a visible connection with the neorectum, which was successfully treated by percutaneous transgluteal drainage. The corresponding healing rates of these treatment approaches are presented in Fig. 3.
In 19 (67.9%) of the above-mentioned 28 patients, it was possible to save the anastomosis without the need for abdominal surgery. Of the 9 remaining patients, 6 eventually needed a resection of the anastomosis, one refrained from further therapy due to metastatic disease, one patient refused any further therapy and the remaining patient received no further therapy because of poor clinical condition.
Continuity
The defunctioning ileostomy could be reversed successfully in 20/30 (67%) patients at the end of follow-up. Corresponding rates of the early EVAC group and late EVAC group were 11/15 (73%) and 9/15 (60%), respectively. Reasons for permanent stoma were chronic sinus in seven patients, refusal to have further surgery in two patients and one patient directly received an end colostomy after initial failure of transanal closure. Median interval from transanal closure to stoma reversal was 175 (72–556) days. Corresponding intervals for the early and late EVAC groups were 175 (72–556) and 165 (78–541) days, respectively.
Quality of life and functionality
The response rates of the EQ-5D-5l, GIQLI and SF-36® at 3, 6, 9 and 12 months were 73, 69, 66 and 86%, respectively. Mean EQ-5D-5L VAS Score at 3 months was 69 (SD 0.22) which increased to 77.9 [SD = 0.25, p < 0.01)] at 12 months. A similar improvement in QoL was seen in the SF-36 scale and GIQLI which is presented in Appendix 1. Response rates of the COREFO were 70, 71 and 100% at 6, 9 and 12 months.
With the lower scores corresponding with less continence disturbance, analysis of the COREFO showed that functionality did not increase from 6 to 12 months postoperative. Response rate of the LARS-score was 80%. Of these responders, 81% experienced Major LARS, 13% Minor-LARS and 6% No LARS (Appendix 2).
Cost analysis
Direct medical costs related to vacuum-assisted early closure
The total direct medical costs related to the vacuum-assisted early closure strategy from start of EVAC to a healed anastomosis or strategy switch from the minimal invasive treatment strategy were €8933 (95% CI 7268–10,707) per patient. The endoscopic examinations to place the Endosponges® and to monitor the healing of the anastomosis contributed the most to the overall cost-burden with median costs of €1539 (616–6773) per patient. The price of one Endosponge® set was €195, contributing to a median €990 (396–6142) per patient. Compared to earlier communicated results from the ‘wait and see’ strategy in our institution, vacuum-assisted early transanal closure showed a 20% increase in healed anastomoses at the end of follow-up [10]. This resembles a number needed to treat of five. This means that five patients have to be treated with vacuum-assisted early transanal closure in order to save one extra anastomosis compared to the ‘wait and see’ strategy.
Discussion
Only half of the leaking anastomosis treated with vacuum-assisted early transanal closure had healed at 6 months of follow-up. This was rather disappointing considering the high healing rate of leaking ileoanal anastomoses at 6 months follow-up using the same technique [14].
There are several reasons why this EVAC technique is less successful in colorectal/anal anastomoses than in ileoanal anastomoses (IPAA). First, time interval before initiation of the EVAC was substantially longer (median of 23 days). Most of the patients were referred after a delayed diagnosis of the leakage. Starting too late with EVAC after the primary operation will probably result in fibrotic bowel ends of the leaking anastomosis in such a way that primary closure is more difficult to achieve. This was demonstrated by a difference in success rate between EVAC started before three weeks and after three weeks. In our previous communication on EVAC for leaking IPAA, all patients had their primary surgery in our hospital and therefore a rapid initiation of EVAC after establishment of the diagnosis was possible in nearly all patients. EVAC was commenced after a median of 2 days from diagnosis of leakage in our previous publication compared to a median of 9 days in the CLEAN study cohort.
The second factor causing lower healing rates was that most of the patients with colorectal anastomoses were treated with neoadjuvant radiotherapy, an important cause for disturbed wound healing [24]. Parallel to the impaired wound healing of perineal wounds following abdominoperineal resection, neoadjuvant radiotherapy seems to have a high impact on secondary healing of the anastomosis. The induced cell-death, vascular damage and associated fibrosis all contribute to lower healing rates [24, 25].
Thirdly, it is technically easier to close a leaking IPAA compared to a colorectal/anal anastomosis. The former is easier to access transanally. In this respect, a technical learning curve has to be appreciated in this study with respect to skills, the approach and the type of sutures that are used. However, at the end of follow-up, 70% of the anastomoses healed which is 20% better than the figures found in an earlier published ‘wait and see’ cohort, as well as the Dutch TME trial [10, 13]. The ‘wait and see’ strategy was associated with a high rate of readmissions taking an average of 22 days, whereas in the present cohort the same figure was only 6 days (including readmissions for stoma reversal). This highlights an important benefit of the vacuum-assisted early transanal closure strategy as it facilitates a better local control of the pelvic sepsis. By early sanitising the presacral abscess cavity, the risk on long-term complications requiring readmission seems to be reduced.
A population-based study in the Netherlands demonstrated that the chronic sinus rate following anastomotic leak is around 48% (unpublished data), which is similar to the non-reversal rate of secondary stoma’s in the TME trial. In the early EVAC group, this rate was 21%, and even though our sample size is small, this reduction in chronic sinus rate of 50% is at least promising. On the other hand, the sinus rate of 47% in the late EVAC group is still alarming and questions the applicability of vacuum-assisted early closure after delayed diagnosis of anastomotic leakage.
The primary closure strategy enabled even patients with major dehiscence of the anastomosis (270 or even 360 degrees) to be healed at the end of follow-up due to approximation of the bowel ends which facilitates secondary healing (Fig. 4). A functional anastomosis would have never been possible in such cases without the early surgical intervention, considering the size of the defect. Furthermore, historical data from our institute indicate that the time to heal when applying a ‘wait and see’ strategy doubles when compared to the present cohort [10].
Patient with a large dehiscence of the anastomosis that underwent successful treatment. A First sigmoidoscopy showing a 270 degrees dehiscence of the anastomosis with a transanal drain that was placed in the referring hospital. B Sigmoidoscopy image after two Endosponge® procedures, showing granulation tissue with pus on the right side of the descending colon. C Image after the fifth Endosponge® procedure showing a clean cavity with granulation tissue. D Two weeks follow-up sigmoidoscopy after transanal closure showing a reduced dehiscence, but with a residual defect. E Small residual sinus after a total of 8 Endosponge® exchanges for a residual defect after transanal closure. F Sigmoidoscopy two week after the last Endosponge® procedure, showing a healed anastomosis
Since the publication of Weidenhagen et al., multiple retrospective studies have published results of the EVAC management of anastomotic leaks [16, 17, 26,27,28]. Anastomotic healing rates ranged between 56 and 97%, the majority being higher than our reported 70%. In contrast to these studies, our cohort solely consists of patients that had full TME with a low anastomosis rather than partial mesorectal excision and more than 70% had preoperative radiotherapy.
An important limitation of the present study is the risk of selection bias. The period needed to include 30 patients was rather long, despite the CLEAN study had been presented at several national meetings and surgeons were invited to refer their leakages through different types of communications. Maybe not all potential study candidates were counselled and included in the study. Most likely, the surgeons referred their worst cases for inclusion expecting no healing by applying a wait and see policy. Another limitation is the lack of a comparative group. This cohort study was designed as proof of concept of a technique that has been shown to be successful for another indication, with the ability of historical comparison with published results from the ‘wait and see’ strategy.
The minimally invasiveness of EVAC, the low-associated costs and the low risks associated with the transanal closure procedure itself, advocate vacuum-assisted early transanal closure as a first step in a proactive and step-up approach of anastomotic leak management. In case no anastomotic healing is reached with vacuum-assisted transanal closure, eventually a redo anastomotic pull-through procedure can be considered [29]. Continuity after redo surgery can be reached in approximately 70–80% of the patients [29, 30]. Another slightly less complex management of the chronic sinus is performing an intersphincteric completion proctectomy with omentoplasty and a permanent end colostomy [12].
This step-up approach of the anastomotic leak is an extensive treatment strategy that is demanding for patients and physicians. Therefore it is of pivotal importance to include the patient in the decision-making process in each of these steps. Patients should be well informed on the increased risk on intra- and postoperative complications of the surgical procedures itself, but especially on the risk of impaired postoperative functionality of the neorectum. Anastomotic leakage, redo surgery and neoadjuvant therapy are responsible for even more LARS than the procedure is causing itself. This was shown in our cohort as a major LARS-score was observed in those who had preserved bowel continuity.
In conclusion, this first prospective study on vacuum-assisted early closure of a leaking anastomosis following TME surgery and 73% neoadjuvant radiotherapy showed that acceptable anastomotic healing and stoma reversal rates can be achieved with this treatment strategy. However, earlier diagnosis of the anastomotic leak and initiation of EVAC as soon as possible might still improve the success rate of the technique.
References
Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1(8496):1479–1482
McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC (2015) Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 102(5):462–479
Vermeer TA, Orsini RG, Daams F, Nieuwenhuijzen GA, Rutten HJ (2014) Anastomotic leakage and presacral abscess formation after locally advanced rectal cancer surgery: incidence, risk factors and treatment. Eur J Surg Oncol 40(11):1502–1509
Trencheva K, Morrissey KP, Wells M, Mancuso CA, Lee SW, Sonoda T et al (2013) Identifying important predictors for anastomotic leak after colon and rectal resection: prospective study on 616 patients. Ann Surg 257(1):108–113
Hallbook O, Sjodahl R (1996) Anastomotic leakage and functional outcome after anterior resection of the rectum. Br J Surg 83(1):60–62
Maggiori L, Bretagnol F, Lefevre JH, Ferron M, Vicaut E, Panis Y (2011) Conservative management is associated with a decreased risk of definitive stoma after anastomotic leakage complicating sphincter-saving resection for rectal cancer. Colorectal Dis 13(6):632–637
Nesbakken A, Nygaard K, Lunde OC, Blucher J, Gjertsen O, Dullerud R (2005) Anastomotic leak following mesorectal excision for rectal cancer: true incidence and diagnostic challenges. Colorectal Dis 7(6):576–581
Peeters KC, Tollenaar RA, Marijnen CA, Klein Kranenbarg E, Steup WH, Wiggers T et al (2005) Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg 92(2):211–216
Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF et al (2011) Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 254(6):868–875
van Koperen PJ, van der Zaag ES, Omloo JM, Slors JF, Bemelman WA (2011) The persisting presacral sinus after anastomotic leakage following anterior resection or restorative proctocolectomy. Colorectal Dis 13(1):26–29
Sloothaak DA, Buskens CJ, Bemelman WA, Tanis PJ (2013) Treatment of chronic presacral sinus after low anterior resection. Colorectal Dis 15(6):727–732
Musters GD, Borstlap WA, Bemelman WA, Buskens CJ, Tanis PJ (2016) Intersphincteric completion proctectomy with omentoplasty for chronic presacral sinus after low anterior resection for rectal cancer. Colorectal Dis 18(2):147–154
den Dulk M, Smit M, Peeters KC, Kranenbarg EM, Rutten HJ, Wiggers T et al (2007) A multivariate analysis of limiting factors for stoma reversal in patients with rectal cancer entered into the total mesorectal excision (TME) trial: a retrospective study. Lancet Oncol 8(4):297–303
Gardenbroek TJ, Musters GD, Buskens CJ, Ponsioen CY, D’Haens GR, Dijkgraaf MG et al (2015) Early reconstruction of the leaking ileal pouch-anal anastomosis: a novel solution to an old problem. Colorectal Dis 17(5):426–432
Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW (2008) Endoluminal vacuum therapy for the treatment of anastomotic leakage after anterior rectal resection. Rozhl Chir 87(8):397–402
van Koperen PJ, van Berge Henegouwen MI, Rosman C, Bakker CM, Heres P, Slors JF et al (2009) The Dutch multicenter experience of the endo-sponge treatment for anastomotic leakage after colorectal surgery. Surg Endosc 23(6):1379–1383
Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW (2008) Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 22(8):1818–1825
Walters SJ, Brazier JE (2005) Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 14(6):1523–1532
Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R et al (1998) Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol 51(11):1055–1068
Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmulling C, Neugebauer E et al (1995) Gastrointestinal quality of life index: development, validation and application of a new instrument. Br J Surg 82(2):216–222
Bakx R, Sprangers MA, Oort FJ, van Tets WF, Bemelman WA, Slors JF et al (2005) Development and validation of a colorectal functional outcome questionnaire. Int J Colorectal Dis 20(2):126–136
Juul T, Ahlberg M, Biondo S, Emmertsen KJ, Espin E, Jimenez LM et al (2014) International validation of the low anterior resection syndrome score. Ann Surg 259(4):728–734
CVZ. Handleiding voor kostenonderzoek (Manual for Cost Research). College voor zorgverzekeringen, Rotterdam
Bullard KM, Trudel JL, Baxter NN, Rothenberger DA (2005) Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum 48(3):438–443
Musters GD, Sloothaak DA, Roodbeen S, van Geloven AA, Bemelman WA, Tanis PJ (2014) Perineal wound healing after abdominoperineal resection for rectal cancer: a two-centre experience in the era of intensified oncological treatment. Int J Colorectal Dis 29(9):1151–1157
Arezzo A, Verra M, Passera R, Bullano A, Rapetti L, Morino M (2015) Long-term efficacy of endoscopic vacuum therapy for the treatment of colorectal anastomotic leaks. Dig Liver Dis 47(4):342–345
Kuehn F, Janisch F, Schwandner F, Alsfasser G, Schiffmann L, Gock M et al (2016) Endoscopic vacuum therapy in colorectal surgery. J Gastrointest Surg 20(2):328–334
Keskin M, Bayram O, Bulut T, Balik E (2015) Effectiveness of endoluminal vacuum-assisted closure therapy (Endosponge) for the treatment of pelvic anastomotic leakage after colorectal surgery. Surg Laparosc Endosc Percutaneous Tech 25(6):505–508
Borstlap WA, Harran N, Tanis PJ, Bemelman WA (2016) Feasibility of the TAMIS technique for redo pelvic surgery. Surg Endosc 30(12):5364–5371
Pitel S, Lefevre JH, Tiret E, Chafai N, Parc Y (2012) Redo coloanal anastomosis: a retrospective study of 66 patients. Ann Surg. 256(5):806–810 discussion 10-1
Funding
This study was funded by Achmea Health Care Insurance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
W.A.A. Borstlap, G.D. Musters, L.P.S. Stassen, H.L. van Westreenen, D. Hess, S. van Dieren, S. Festen, E.J. van der Zaag, P.J. Tanis and W.A. Bemelman have no conflicts of interest or financial ties to disclose.
Appendices
Appendix 1: Quality of life assessment
EQ-5D-5L | 3 m (n = 19) | 6 m (n = 20) | 9 m (n = 18) | 12 m (n = 24) |
|---|---|---|---|---|
EQ-5D index score (mean sd) | 0.69 (0.22) | 0.79 (0.14) | 0.80 (0.17) | 0.80 (0.25) |
EQ-5D VAS score | 67.22 (20.50) | 70.26 (14.07) | 76.39 (17.05) | 77.91 (16.64) |
SF-36 | 3 m (n = 19) | 12 m (n = 25 | p value* |
|---|---|---|---|
Physical functioning (mean, SD) | 60 (20) | 75 (26) | 0.031 |
Role Physical (mean, SD) | 25 (35) | 66 (46) | 0.003 |
Bodily Pain (mean, SD) | 69 (24) | 74 (29) | 0.312 |
Social functioning (mean, SD) | 53 (34) | 71(30) | 0.044 |
Mental health (mean, SD) | 71 (25) | 78 (19) | 0.171 |
Role emotional (mean, SD) | 53 (47) | 68 (47) | 0.335 |
Vitality (mean, SD) | 55 (22) | 65 (26) | 0.162 |
General health perception (mean, SD) | 59 (19) | 63 (25) | 0.569 |

Appendix 2: Treatment-dependent direct medical costs per patient of both treatment methods in Euros
Cost analysis | Price per unit (€) | Units | Costs |
|---|---|---|---|
Costs made from diagnosis of leakage to healed anastomosis or strategy switch (n = 30) | |||
Hospital admittance (day) following transanal closure1,a | 476 | 3 (1–19) | 1190 (476–9044) |
Total hospital days for readmittance in post EVAC courseb | 476 | 0 (0–21) | 0 (0–9996) |
Endosponge set (per change)2 | 195 | 5 (2–31) | 990 (396–6142) |
Total amount of transanal closures3 | 1413 | 1 (1–3) | 1436 (1436–4307) |
Sigmoidoscopy4 | 202 | 7.5 (3–33) | 1539 (616–6773) |
CT-abdomen5 | 212 | 2 (0–4) | 431 (0–862) |
Colonogram6 | 150 | 0 (0–6) | (0–902) |
Outpatients clinic visits | 113 | 0.5 (0–14) | 57 (0–1584) |
ER room visits7 | 259 | 0 (0–2) | 0 (0–518) |
Reinterventions# | 378–1915 | 1 (1–2) | 1096 (149–1916) |
Total costsd | Mean (CI) : 8933-(7268–10707) | ||
CLEAN costs including stoma reversal, redo procedure or proctectomy with end colostomy (n = 30) | |||
Total hospital days for readmittance in post EVAC course | 476 | 6 (0–47) | 2856 (0–22,372) |
Endosponge set (per change)2 | 195 | 5 (2–31) | 991 (396–6142) |
Sigmoidoscopy4 | 202 | 8 (3–34) | 1642 (616–6978) |
CT-abdomen5 | 212 | 2 (0–7) | 431 (0–1508) |
Colonogram6 | 150 | 0 (0–6) | 0 (0–902) |
Outpatients clinic visits | 113 | 1 (0–16) | 113 (0–1810) |
ER room visits7 | 259 | 0 (0–2) | 0 (0–518) |
Reinterventions# | 378–1915 | 2 (0–7) | 1345 (149–3622) |
Redo procedure | 5440 | 0 (0–2) | 0 (2–10,880) |
Stoma reversal@ | 2504 | 1 (0–2) | 2504 (0–6950) |
Total costsd | Mean (CI): 17,018 (13,822–20,832) | ||
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Borstlap, W.A.A., Musters, G.D., Stassen, L.P.S. et al. Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surg Endosc 32, 315–327 (2018). https://doi.org/10.1007/s00464-017-5679-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-017-5679-6