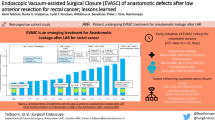
Abstract
Dehiscence of colorectal anastomosis is a serious complication that is associated with increased mortality, impaired functional and oncological outcomes. The hypothesis was that anastomosis reinforcement and vacuum trans-anal drainage could eliminate some risk factors, such as mechanically stapled anastomosis instability and local infection. Patients with rectal cancer within 10 cm of the anal verge and low anterior resection with double-stapled technique were included consecutively. A stapler anastomosis was supplemented by trans-anal reinforcement and vacuum drainage using a povidone-iodine-soaked sponge. Modified reinforcement using a circular mucosa plication was developed and used. Patients were followed up by postoperative endoscopy and outcomes were acute leak rate, morbidity, and diversion rate. The procedure was successfully completed in 52 from 54 patients during time period January 2019–October 2020. The mean age of patients was 61 years (lower–upper quartiles 54–69 years). There were 38/52 (73%) males and 14/52 (27%) females; the neoadjuvant radiotherapy was indicated in a group of patients in 24/52 (46%). The mean level of anastomosis was 3.8 cm (lower–upper quartiles 3.00–4.88 cm). The overall morbidity was 32.6% (17/52) and Clavien–Dindo complications ≥ 3 grade appeared in 3/52 (5.7%) patients. No loss of anastomosis was recorded and no patient died postoperatively. The symptomatic anastomotic leak was recorded in 2 (3.8%) patients and asymptomatic blind fistula was recorded in one patient 1/52 (1.9%). Diversion ileostomy was created in 1/52 patient (1.9%). Reinforcement of double-stapled anastomosis using a circular mucosa plication with combination of vacuum povidone-iodine-soaked sponge drainage led to a low acute leak and diversion rate. This pilot study requires further investigation.
Registered at ClinicalTrials.gov.: Trial registration number is NCT04735107, date of registration February 2, 2021, registered retrospectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rectal resection for cancer is still associated with considerable morbidity. Acute leak (AL) is probably the most serious complication and is associated with increased postoperative mortality, with long-term consequences, such as a negative impact on function and oncological outcomes [1,2,3]. A proportion of patients with AL end up with an unplanned definitive stoma [4]; and increased economic costs associated with the treatment of complications and prolonged stays in the ICU should be taken in account [5, 6].
Published data on the occurrence of this complication are heterogeneous and greatly depend on the definition used, the design of the study [7,8,9] the duration of the study, and the composition of the group of patients studied [10, 11]. Very often, leaks that are asymptomatic [11] and leaks that are diagnosed after stoma closure are not accurately reported. The incidence of AL can reach 20–30% [2, 12]; and, if we only focus on the group of patients operated on for middle and lower rectal cancer, the leak rate may be even higher.
Many papers have been published analyzing preoperative, intraoperative, and postoperative factors associated with the development of AL [13,14,15]. Male gender, neoadjuvant radiotherapy, and low localization of anastomosis are generally accepted risk factors for AL development [16]. Surgeon experience is another factor which plays a very important role, and not much is written about it [17]. Surgeon experience is difficult to measure and adopt into risk calculation modeling; however, surgeons in high-volume centers present AL rates below 5% [18] and even these satisfactory results are associated with a high diversion rate [16, 19].
The scientific literature is yet to confirm a preference or differentiate between open, laparoscopic, robotic, or trans-anal approaches in the reduction of the incidence of this unpleasant complication [20,21,22]. Similarly, research in the field of leak prevention focuses in many directions [23]: stapled anastomosis replacement or modification [24, 25]; intraluminal biodegradable sheath application [26]; the colon microbiome influence [27,28,29]; and postoperative de-tension of the colon above an anastomosis [30, 31]. Various types of anastomosis reinforcement [32,33,34,35,36,37] have also been tested experimentally and clinically.
We investigated the intraoperative factors associated with AL that may be preventable. We initially needed to determine accurate leak rates in our patient group, including asymptomatic leaks. Therefore, the aims of our project were:
-
1. To determine the occurrence of an acute leak in patients operated on for rectal cancer up to 10 cm. Further, to specify the location and morphology of stapler line disruption in patients diagnosed with AL [38].
-
2. To verify the effect of modified trans-anal reinforcement in combination with trans-anastomotic vacuum drainage on the occurrence of acute leaks.
Materials and methods
The study was approved by the ethics committee of our institution (Jessenius Medical Faculty in Martin, Slovak Republic) and was conducted in accordance with the Declaration of Helsinki.
Inclusion criteria
All patients provided written informed consent. The study included consecutive patients older than 18 years who had low anterior resection of the rectum and anastomosis performed by double-stapler technique, for rectal cancer located within 10 cm from the anal verge (Fig. 1).
All patients had undergone standard preoperative diagnostic evaluation, e.g., colonoscopy, endorectal sonography, or pelvic magnetic resonance imaging. Nutrition screening was performed in all patients. If patients had undergone neoadjuvant chemoradiotherapy (CHRT), restaging was performed within 6 weeks of CHRT completion, and surgery was performed 10 weeks after CHRT completion. For the surgical procedure, low anterior resection (LAR) was performed by experienced surgeons who perform more than 50 rectal procedures per year and have sufficient expertise in minimally invasive surgery. Oral bowel preparation was used preoperatively and antibiotics were administered according to protocol.
Surgical technique
The procedure standard (descending colon blood perfusion, tension-free anastomosis, safely performed stapled anastomosis and reinforcement, and safely performed mucosal flap) was defined. Simultaneous checkpoints to control milestones were identified and methodology of their documentation (video, photography) was defined. The purpose was to achieve demonstrable control over the individual steps during the surgical procedure (Table 1).
Laparoscopic procedures were performed in the Lloyd-Davies position, using the 4-port technique. During the abdominal phase, dissection was guided by a medio-lateral approach. A high tie of the AMI was performed in all patients. Dissection was performed medio-laterally and down to the pelvic floor according to the principles of TME. The rectum was transected using an endostapler after lavage with Betadine solution (Egis Pharmaceuticals, PLS, Budapest, Hungary). Furthermore, the splenic flexure was fully mobilized using a combination of medio-lateral and lateral approaches. In most cases, the inferior mesenteric vein was divided.
The marginal artery was dissected and the character of arterial blood flow was carefully evaluated; pulsatile arterial blood flow was considered as sign of adequate colon perfusion (Checkpoint 1).
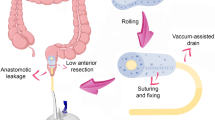
A specimen of tumor was pulled through the mini-laparotomy and resected. The descending colon was divided at the level of the distal part and the colonic mucosa was again evaluated with respect to blood perfusion; a light red or pink colored mucosa and fresh light red capillary bleeding were considered as signs of good colonic mucosa perfusion (Checkpoint 2). The colon needed to lie freely in the sacrum excavation and no tension was allowed on the mesenteric site. This was confirmed by lifting the colon ventrally from the sacrum at the promontory level after anastomosis construction (Checkpoint 3). The anastomosis was performed end-to-end using a double-stapler technique, strictly between the descending colon and rectum in a tension-free manner. A pelvic drain was left in place till the third postoperative day.
Trans-anal phase
As part of the trans-anal phase, a Lone Star retractor (Cooper Surgical, Inc. USA) and a plastic single use anoscope were applied. An initial, careful inspection and manual check of the stapler anastomosis integrity, the blood supply to the colonic mucosa, and signs of a tension-free anastomosis were performed (Fig. 2) (Checkpoint 4). The mucosal flap/plication was subsequently created using individual PDS II 5/0 sutures (polydiaxonone, Ethicon, Johnson & Johnson, USA): individual stitches were placed on each quadrant; and then another four stitches were applied in between (Fig. 3). It is important to note that the condition of the mucosal flap upon creation were signs of a floppy, prolapsing colonic wall into the anastomosis. Finally, a sponge soaked (Endo-SPONGE, B. Braun, Germany) with povidone-iodine (Betadine, Egis Pharmaceuticals, PLS, Budapest, Hungary) was introduced into the anastomosis (negative pressure 80–100 mm Hg). The trans-anal sponge drain was removed 24 h postoperatively.
Fecal diversion
The decision on diversion was based on intraoperative checkpoint adherence: when Checkpoint 6 and 7 were not fulfilled, an ileostomy was created.
Follow-up
The data regarding the type of procedure, type of anastomosis, stapler diameter, the number of stapler cartridges used, dissection of the mesenteric blood vessels, and complete histopathology were collected prospectively. CRP levels were assessed on the third and fifth day after surgery.
Patients were followed up for 3 months, and postoperative endoscopy was performed before discharge, usually on postoperative day 7, 1 month after surgery, and 3 months after surgery.
Statistics
The data were explored and analyzed in R [38], ver. 4.0.2. The number of patients at a level of a factor, together with the percentage of patients at the level of the factor was used to summarize the data.
Results
Patients with mid- and low-rectal cancer were included in the study (Table 2). The mean age of patients was 61 years (lower–upper quartiles 54–69 years). There were 38/52 (73%) males and 14/52 (27%) females; the neoadjuvant radiotherapy was indicated in a group of patients in 24/52 (46%). The mean level of anastomosis was 3.83 cm (lower–upper quartiles 3.00–4.88 cm) from anal verge and 2.00 cm (lower–upper quartiles 1.00–2.00 cm) from upper edge of sphincters. All operations were performed minimally invasively (100%). The overall mean operative time was 255 min (lower–upper quartiles 225–277 min), the mean perineal phase time was 25 min (lower–upper quartiles 20–30 min).
Morbidity and mortality
No patients died postoperatively and no intraoperative complication was recorded. Endoscopic examination before discharge home was completed in all 52 patients (100%). Patients underwent control endoscopy 1 month (50/52 patients) and 3 months (49/52 patients) after surgery. The overall morbidity was 33% (17/52) and serious complications, Clavien–Dindo ≥ 3 grade, appeared in 3/52 (5.7%) patients (Table 3). No loss of anastomosis was recorded, 3 (5.7%) anastomotic complications (2 symptomatic, 1 asymptomatic) were recorded, at all. The symptomatic anastomotic leak was recorded in 2/52 (3.8%) patients, one acute leak a one recto-vaginal fistula. A recto-vaginal fistula was diagnosed 1 month after surgery in one female. A retrospective analysis of the recorded video revealed a technical error of the surgeon in performing the stapler anastomosis. Another patient was diagnosed with asymptomatic blind chronic anastomotic fistula 3 months after surgery, which did not require any treatment intervention.
Diversion ileostomy was placed on 1/52 (1.9%) patient. This ileostomy was performed due to uncertainty about the quality of the reinforcement performed (Checkpoints 6 and 7).
Protocol violation and patient exclusion
A low anterior resection with double-stapled anastomosis for extraperitoneal rectal cancer was indicated in 54 of 186 patients (Fig. 1). In two patients, no reinforcement was performed due to anal canal stenosis after hemorrhoid surgery, and in another patient due to obesity (BMI 42). One of these patients developed AL, which was treated by ileostomy and trans-anal vacuum drainage. The standardized protocol was violated in one patient; the colon was not adequately cleaned before the surgery. Immediately after the operation a massive defecation of stool with a temporary obstruction of the colon above the sponge occurred. This patient was excluded from the study. Acute B grade leak was observed and treated with trans-anal vacuum drainage in the postoperative period. The patient did not lose the anastomosis.
Discussion
We showed that reinforcement of a double-stapled anastomosis using mucosal flap with the combination of vacuum povidone-iodine-soaked sponge drainage led to a significant decrease in AL and diversion rate. However, AL is a complication that still deserves considerable attention.
The search for optimal treatments should focus on rapid pelveoperitonitis or peritonitis treatment and the rescue of sphincters, if possible. No less important is the search for factors associated with the leak formation. Analysis of the photographic documentation of endoscopic findings on the anastomosis from our previous study [38] allowed us to observe signs of local stress on the anastomosis, ischemic changes, loose stapler clamps, as well as other pathological findings, such as fibrosis and inflammatory polyps. These signs suggest a healing disorder and “restlessness” in the area of the stapler anastomosis, which is associated with local ischemia or local infection. The result is a defect in the anastomosis with the spread of infection to the perianastomotic space or an exacerbated and scarring reaction. These findings led us to our hypothesis formulation. Five basic pathogenic moments were identified:
-
1. The blood supply to the large intestine, and especially the section above the anastomosis, is very important and must be verified intraoperatively.
-
2. Tension-free colorectal anastomosis is an indispensable condition for successful completion of the procedure.
-
3. Double-stapler anastomosis poses a higher risk of mechanical disruption of the stapler line; therefore, trans-anal reinforcement of the anastomosis may play an important role in its prevention.
-
4. A colorectal anastomosis is a contaminated wound and is at risk of bacterial invasion during the first 24 h, like any other contaminated wound.
-
5. Endo-anal trans-anastomotic drainage may play a role in reducing the risk of leakage and de-tension of the colon above the anastomosis.
If we pause at the first two points of our hypothesis, there will probably be general agreement that blood flow to the colon and tension-free anastomosis are very important; however, it should be emphasized that tension-free anastomosis requires full mobilization of the splenic flexure, in contrast to resection of upper rectal tumors. This is consistent with Rink et al., who published the Delphi Consensus from the German expert meeting in 2020 [16]. However, full mobilization of the splenic flexure is technically demanding, sometimes significantly prolonging the operation, and is associated with risk of injury to the spleen, pancreas, or marginal collateral vessel. Surgeon training seems to be very important in this instance.
Mobilization of the splenic flexure requires division of the main vessels, AMI, and often the inferior mesenteric vein (VMI). This leads to a decrease in arterial perfusion [39], but also to poor left colon venous drainage. Another alternative to high AMI is division of the AMI distal to the origin of the left colic artery (LCA) (the so-called low AMI tie), which would ensure sufficient blood supply to the sigmoid colon. In this context, Guo et al. directly measured the pressure in the LCA and found that the mean arterial pressure in the ACS at low AMI ligation is higher than at high AMI ligation [40]. LCA dissection may be an alternative, especially in at-risk patients with sclerotic arterial disease. The question is whether dissection of the apical nodes is necessary and will be comparatively radical. At present, this method is not accepted as a standard and is the subject of further evaluation. Consideration of magistral vessel ligature is very important and knowledge of the anatomical variability of the vascular supply to the colon is necessary. The medial colic artery (MCA), which is the source of blood flow for ACS, may be completely absent in a large proportion of patients [41]. The medial collateral artery (Arc of Riolan), which accompanies VMI, occurs in about 7% of patients, and might significantly complicate full splenic flexure mobilization. Its interruption might lead to severe ischemia of the left colon. Intraoperative identification of vascular variability is difficult and often impossible in obese patients.
Therefore, to gain control over the blood supply to the large intestine, checkpoints have been set in our standard (Table 2). We relied on old surgical principles: control of pulsatile flow on the marginal artery and control of the mucosa and its blood supply. Although we have infrared camera technology, we have not used it as the standard in our study. The interpretation probably requires a quantitative approach [42], especially in patient with VMI division and impaired venous blood drainage of the left colon.
As regards the next point of our hypothesis, the double-stapling technique of colorectal anastomosis, previously published work on the topic of reinforcement and modifications of the stapler anastomosis [25, 43] may indirectly indicate doubts about the safety of this technique [44, 45]. Reinforcement of a stapled or double-stapled anastomosis of various technical designs is appearing with increasing frequency in the literature and remains the subject of ongoing studies. Individual authors have used various techniques, such as glues [33, 36], bio absorbable pads [34], intracorporeal applied sutures [32], or trans-analy applied sutures [35, 37, 46]. We started with trans-anal suture reinforcement within our hypothesis as we believed that the reinforcement suture would relieve local tension between the anastomosed colon and the rectum. We subsequently discovered that the transmurally applied sutures technique is difficult and can lead to injuries to the wall of the colon and rectum around the anastomosis. Therefore, we modified this technique. The mucosal flap was created with the assumption that well-perfused tissue, rich with immunocompetent cells, covers entry to the wound and decrease risk of bacterial deep invasion. This might lead to local inter-staple infection reduction in the first 24 h after surgery. Importantly, however, is that the mucosal flap should be performed with respect to the tension-free technique.
The last pillar of our hypothesis is vacuum drainage using a sponge infused with an antiseptic solution. This part of the hypothesis is based on several findings. A colorectal anastomosis is classified as a contaminated wound. The first (exudative) phase of healing is characterized by the lack of immunocompetent cells, neutrophils, in the wound which provide immune protection against bacterial invasion. The wound is impermeable to bacteria after 24 h [47]. Therefore, the reduction of bacterial load at the time of wound formation may be important in the development of local infection between the stapler clamps [27].
Another pathophysiological consideration is the intraluminal pressure in the large intestine above the anastomosis. Intestinal decompression above the anastomosis may reduce the risk of AL [30, 31]. Endosponge is used for intraluminal treatment of acute leakage [48], but its preventive use in this indication is no longer fully known. Therefore, we must rely on data from studies where vacuum therapy has been tested in the prevention of early infection [49,50,51]. However, the conclusions are ambiguous and it cannot be argued that vacuum therapy should be routinely used to prevent early infection in colorectal surgery [52]. Similarly, we cannot unequivocally say that the applied vacuum leads to a reduction in the bacterial load in the wound area [53]. What is interesting, however, is that vacuum therapy can promote neovascularization, and thus healing. Labler et al., point to higher local concentrations of IL-8 and VEGF, and thus higher leukocyte attraction and improved promotion of neovascularization [51]. Any trans-anal drainage can only be applied to patients with a perfectly prepared and clean colon; otherwise, the unpleasant complication of obstruction may result, as seen in our one patient.
The indication of fecal diversion in rectal resection may not only have medical reasons. Many articles regarding this have been published [19], and to state that ileostomy can alleviate the clinical severity of AL but probably does not prevent it. In our cohort, we indicated ileostomy in one patient out of 52 (1.9%). The reason was the uncertainty about mucosal plication vitality after the completion of reinforcement (hematoma and mucosa crack) (Checkpoints 6 and 7).
Our study had limitations in terms of the number of patients and functional outcomes monitoring. Although the trans-anal phase of the operation did not last longer than 20 min on average, this requires further evaluation.
Our pilot results would imply that standardization of surgery, perioperative control of colonic blood flow, tension-free anastomosis, trans-anal control of anastomosis integrity, and reinforcement with vacuum drainage led to a low acute leak and low diversion rate. Our results from this preliminary study are promising and require further investigation. At present, we are unable to clearly identify the value and weight of each individual step, and believe that this is a combined effect of all individual measures. What needs to be emphasized, however, is that any trans-anal intervention cannot replace proper surgical technique. Surgeon experience is an irreplaceable factor.
Data availability
FERKO, Alexander (2021), “Mucosal Flap Reinforced Colorectal Anastomosis and Trans-Anal Vacuum Drainage”, Mendeley Data, V1. http://dx.doi.org/10.17632/7x93t5hv6p.1.
Code availability
Declared in section Material and methods, page 5 in Manuscript file.
References
Smith JD, Butte JM, Weiser MR, D’Angelica MI, Paty PB, Temple LK et al (2013) Anastomotic leak following low anterior resection in stage IV rectal cancer is associated with poor survival. Ann Surg Oncol 20:2641–2646. https://doi.org/10.1245/s10434-012-2854-9
Hain E, Maggiori L, Manceau G, Mongin C, Prost ADJ, Panis Y (2017) Oncological impact of anastomotic leakage after laparoscopic mesorectal excision. Br J Surg 104:288–295. https://doi.org/10.1002/bjs.10332
Karim A, Cubas V, Zaman S, Khan S, Patel H, Waterland P (2020) Anastomotic leak and cancer-specific outcomes after curative rectal cancer surgery: a systematic review and meta-analysis. Tech Coloproctol 24:513–525. https://doi.org/10.1007/s10151-020-02153-5
Zhou X, Wang B, Li F, Wang J, Fu W (2017) Risk factors associated with nonclosure of defunctioning stomas after sphincter-preserving low anterior resection of rectal cancer: a meta-analysis. Dis Colon Rectum 60:544–554. https://doi.org/10.1097/DCR.0000000000000819
Ashraf SQ, Burns EM, Jani A, Altman S, Young JD, Cunningham C et al (2013) The economic impact of anastomotic leakage after anterior resections in English NHS hospitals: are we adequately remunerating them? Colorectal Dis 15:e190-198. https://doi.org/10.1111/codi.12125
Hammond J, Lim S, Wan Y, Gao X, Patkar A (2014) The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg 18:1176–1185. https://doi.org/10.1007/s11605-014-2506-4
Borly L, Ellebaek MB, Qvist N (2015) Leakage after surgery for rectum cancer: inconsistency in reporting to the danish colorectal cancer group. Surg Res Pract 2015:376540. https://doi.org/10.1155/2015/376540
Olsen BC, Sakkestad ST, Pfeffer F, Karliczek A (2019) Rate of anastomotic leakage after rectal anastomosis depends on the definition: pelvic abscesses are significant. Scand J Surg 108:241–249. https://doi.org/10.1177/1457496918812223
Draginov A, Chesney TR, Quereshy HA, Chadi SA, Quereshy FA (2020) Association of high ligation versus low ligation of the inferior mesenteric artery on anastomotic leak, postoperative complications, and mortality after minimally invasive surgery for distal sigmoid and rectal cancer. Surg Endosc 34:4593–4600. https://doi.org/10.1007/s00464-019-07203-0
Sparreboom CL, van Groningen JT, Lingsma HF, Wouters M, Menon AG, Kleinrensink GJ et al (2018) Different risk factors for early and late colorectal anastomotic leakage in a nationwide audit. Dis Colon Rectum 61:1258–1266. https://doi.org/10.1097/DCR.0000000000001202
Cong ZJ, Hu LH, Bian ZQ, Ye GY, Yu MH, Gao YH et al (2013) Systematic review of anastomotic leakage rate according to an international grading system following anterior resection for rectal cancer. PLoS ONE 8:e75519. https://doi.org/10.1371/journal.pone.0075519
Borstlap WAA, Westerduin E, Aukema TS, Bemelman WA, Tanis PJ, Dutch Snapshot Research Group (2017) Anastomotic leakage and chronic presacral sinus formation after low anterior resection: results from a large cross-sectional study. Ann Surg 266:870–877. https://doi.org/10.1097/SLA.0000000000002429
Meyer J, Naiken S, Christou N, Liot E, Toso C, Buchs NC et al (2019) Reducing anastomotic leak in colorectal surgery: the old dogmas and the new challenges. World J Gastroenterol 25:5017–5025. https://doi.org/10.3748/wjg.v25.i34.5017
Kawada K, Sakai Y (2016) Preoperative, intraoperative and postoperative risk factors for anastomotic leakage after laparoscopic low anterior resection with double stapling technique anastomosis. World J Gastroenterol 22:5718–5727. https://doi.org/10.3748/wjg.v22.i25.5718
Qu H, Liu Y, Bi DS (2015) Clinical risk factors for anastomotic leakage after laparoscopic anterior resection for rectal cancer: a systematic review and meta-analysis. Surg Endosc 29:3608–3617. https://doi.org/10.1007/s00464-015-4117-x
Rink AD, Kienle P, Aigner F, Ulrich A (2020) How to reduce anastomotic leakage in colorectal surgery-report from German expert meeting. Langenbecks Arch Surg 405:223–232. https://doi.org/10.1007/s00423-020-01864-5
Garcia-Granero E, Navarro F, Santacruz CC, Frasson M, Garcia-Granero A, Marinello F et al (2017) Individual surgeon is an independent risk factor for leak after double-stapled colorectal anastomosis: an institutional analysis of 800 patients. Surgery 162:1006–1016. https://doi.org/10.1016/j.surg.2017.05.023
Kim JC, Lee JL, Kim CW, Lim SB, Alsaleem HA, Park SH (2019) Mechanotechnical faults and particular issues of anastomotic complications following robot-assisted anterior resection in 968 rectal cancer patients. J Surg Oncol 120:1436–1445. https://doi.org/10.1002/jso.25765
Garg PK, Goel A, Sharma S, Chishi N, Gaur MK (2019) Protective diversion stoma in low anterior resection for rectal cancer: a meta-analysis of randomized controlled trials. Visc Med 35:156–160. https://doi.org/10.1159/000497168
European Society of Coloproctology Collaborating Group (2018) An international multicentre prospective audit of elective rectal cancer surgery; operative approach versus outcome, including transanal total mesorectal excision (TaTME). Colorectal Dis 20(Suppl 6):33–46. https://doi.org/10.1111/codi.14376
Hajibandeh S, Hajibandeh S, Eltair M, George AT, Thumbe V, Torrance AW et al (2020) Meta-analysis of transanal total mesorectal excision versus laparoscopic total mesorectal excision in management of rectal cancer. Int J Colorectal Dis 35:575–593. https://doi.org/10.1007/s00384-020-03545-7
Guel-Klein S, Biebl M, Knoll B, Dittrich L, Weiss S, Pratschke J et al (2019) Anastomotic leak after transanal total mesorectal excision: grading of severity and management aimed at preservation of the anastomosis. Colorectal Dis 21:894–902. https://doi.org/10.1111/codi.14635
Vallance A, Wexner S, Berho M, Cahill R, Coleman M, Haboubi N et al (2017) A collaborative review of the current concepts and challenges of anastomotic leaks in colorectal surgery. Colorectal Dis 19:O1–O12. https://doi.org/10.1111/codi.13534
Lu Z, Peng J, Li C, Wang F, Jiang W, Fan W et al (2016) Efficacy and safety of a NiTi CAR 27 compression ring for end-to-end anastomosis compared with conventional staplers: a real-world analysis in Chinese colorectal cancer patients. Clinics 71:264–270. https://doi.org/10.6061/clinics/2016(05)04
Crafa F, Smolarek S, Missori G, Shalaby M, Quaresima S, Noviello A et al (2017) Transanal inspection and management of low colorectal anastomosis performed with a new technique: the TICRANT Study. Surg Innov 24:483–491. https://doi.org/10.1177/1553350617709182
Bakker IS, Morks AN, Ten Cate Hoedemaker HO, Burgerhof JGM, Leuvenink HG, van Praagh JB et al (2017) Randomized clinical trial of biodegradeable intraluminal sheath to prevent anastomotic leak after stapled colorectal anastomosis. Br J Surg 104:1010–1019. https://doi.org/10.1002/bjs.10534
Schardey HM, Wirth U, Strauss T, Kasparek MS, Schneider D, Jauch KW (2020) Prevention of anastomotic leak in rectal cancer surgery with local antibiotic decontamination: a prospective, randomized, double-blind, placebo-controlled single center trial. Int J Colorectal Dis 35:847–857. https://doi.org/10.1007/s00384-020-03544-8
Hyoju SK, Klabbers RE, Aaron M, Krezalek MA, Zaborin A, Wiegerinck M et al (2018) Oral polyphosphate suppresses bacterial collagenase production and prevents anastomotic leak due to Serratia marcescens and Pseudomonas aeruginosa. Ann Surg 267:1112–1118. https://doi.org/10.1097/SLA.0000000000002167
Gaines S, Hyoju S, Williamson AJ, van Praagh JB, Zaborina O, Rubin DT et al (2020) Infliximab does not promote the presence of collagenolytic bacteria in a mouse model of colorectal anastomosis. J Gastrointest Surg. https://doi.org/10.1007/s11605-019-04486-5
Matsuda M, Tsuruta M, Hasegawa H, Okabayashi K, Kondo T, Shimada T et al (2016) Transanal drainage tube placement to prevent anastomotic leakage following colorectal cancer surgery with double stapling reconstruction. Surg Today 46:613–620. https://doi.org/10.1007/s00595-015-1230-3
Shigeta K, Okabayashi K, Baba H, Hasegawa H, Tsuruta M, Yamafuji K et al (2016) A meta-analysis of the use of a transanal drainage tube to prevent anastomotic leakage after anterior resection by double-stapling technique for rectal cancer. Surg Endosc 30:543–550. https://doi.org/10.1007/s00464-015-4237-3
Maeda K, Nagahara H, Shibutani M, Ohtani H, Sakurai K, Toyokawa T et al (2015) Efficacy of intracorporeal reinforcing sutures for anastomotic leakage after laparoscopic surgery for rectal cancer. Surg Endosc 29:3535–3542. https://doi.org/10.1007/s00464-015-4104-2
Wenger FA, Szucsik E, Hoinoiu BF, Cimpean AM, Ionac M, Raica M (2015) Circular anastomotic experimental fibrin sealant protection in deep colorectal anastomosis in pigs in a randomized 9-day survival study. Int J Colorectal Dis 30:1029–1039. https://doi.org/10.1007/s00384-015-2260-4
Placer C, Enriquez-Navascues JM, Elorza G, Timoteo A, Mugica JA, Borda N et al (2014) Preventing complications in colorectal anastomosis: results of a randomized controlled trial using bioabsorbable staple line reinforcement for circular stapler. Dis Colon Rectum 57:1195–1201. https://doi.org/10.1097/DCR.0000000000000207
Baek SJ, Kim J, Kwak J, Kim SH (2013) Can trans-anal reinforcing sutures after double stapling in lower anterior resection reduce the need for a temporary diverting ostomy? World J Gastroenterol 19:5309–5313. https://doi.org/10.3748/wjg.v19.i32.5309
Wu Z, Vakalopoulos KA, Kroese LF, Boersema GS, Kleinrensink GJ, Jeekel J et al (2013) Reducing anastomotic leakage by reinforcement of colorectal anastomosis with cyanoacrylate glue. Eur Surg Res 50:255–261. https://doi.org/10.1159/000350383
Altomare DF, Delrio P, Shelgyn Y, Rybakov E, Vincenti L, De Fazio M et al (2021) Transanal reinforcement of low rectal anastomosis versus protective ileostomy after total mesorectal excision for rectal cancer. Preliminary results of a randomized clinical trial. Colorectal Dis. https://doi.org/10.1111/codi.15685
Ferko A, Rejholoc J, Škrovina M, Tachecí I, Sirák I (2020) Colorectal anastomosis dehiscence: a call for more detailed morphological classification. Wideochir Inne Tech Maloinwazyjne. https://doi.org/10.5114/wiitm.2020.97367
Son GM, Kim TU, Park BS, Jung HJ, Lee SS, Yoon JU et al (2019) Colonic hypoperfusion following ligation of the inferior mesenteric artery in rectosigmoid colon cancer patients. Ann Surg Treat Res 97:74–82. https://doi.org/10.4174/astr.2019.97.2.74
Guo Y, Wang D, He L, Zhang Y, Zhao S, Zhang L et al (2017) Marginal artery stump pressure in left colic artery-preserving rectal cancer surgery: a clinical trial. ANZ J Surg 87:576–581. https://doi.org/10.1111/ans.13032
Sakorafas GH, Zouros E, Peros G (2006) Applied vascular anatomy of the colon and rectum: clinical implications for the surgical oncologist. Surg Oncol 15:243–255. https://doi.org/10.1016/j.suronc.2007.03.002
Son GM, Kwon MS, Kim Y, Kim J, Kim SH, Lee JW (2019) Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg Endosc 33:1640–1649. https://doi.org/10.1007/s00464-018-6439-y
Tulchinsky H, Kashtan H, Rabau M, Wasserberg N (2010) Evaluation of the NiTi shape memory BioDynamix ColonRing in colorectal anastomosis: first in human multi-center study. Int J Colorectal Dis 25:1453–1458. https://doi.org/10.1007/s00384-010-0985-7
Braunschmid T, Hartig N, Baumann L, Dauser B, Herbst F (2017) Influence of multiple stapler firings used for rectal division on colorectal anastomotic leak rate. Surg Endosc 31:5318–5326. https://doi.org/10.1007/s00464-017-5611-0
Kim JH, Kim HY, Lee IK, Oh ST, Kim JG, Lee YS (2015) Intra-operative double-stapled colorectal or coloanal anastomotic complications of laparoscopic low anterior resection for rectal cancer: double-stapled anastomotic complication could result in persistent anastomotic leakage. Surg Endosc 29:3117–3124. https://doi.org/10.1007/s00464-014-4035-3
Vallicelli C, Pirrera B, Alagna V, Fantini E, Palini GM, Zanini N et al (2020) Intraoperative endoscopy with immediate suture reinforcement of the defect in colorectal anastomosis: a pilot study. Updates Surg. https://doi.org/10.1007/s13304-020-00746-1
Velnar T, Bailey T, Smrkolj V (2009) The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res 37:1528–1542. https://doi.org/10.1177/147323000903700531
Borstlap WAA, Musters GD, Stassen LPS, van Westreenen HL, Hess D, van Dieren S et al (2018) Vacuum-assisted early transanal closure of leaking low colorectal anastomoses: the CLEAN study. Surg Endosc 32:315–327. https://doi.org/10.1007/s00464-017-5679-6
Javed AA, Teinor J, Wright M, Ding D, Burkhart RA, Hundt J et al (2019) Negative pressure wound therapy for surgical-site infections. Ann Surg 269:1034–1040. https://doi.org/10.1097/sla.0000000000003056
Murphy PB, Knowles S, Chadi SA, Vogt K, Brackstone M, Koughnett JAV et al (2019) Negative pressure wound therapy use to decrease surgical nosocomial events in colorectal resections (NEPTUNE): a randomized controlled trial. Ann Surg 270:38–42. https://doi.org/10.1097/SLA.0000000000003111
Labler L, Rancan M, Mica L, Harter L, Mihic-Probst D, Keel M (2009) Vacuum-assisted closure therapy increases local interleukin-8 and vascular endothelial growth factor levels in traumatic wounds. J Trauma 66:749–757. https://doi.org/10.1097/TA.0b013e318171971a
Kuper TM, Murphy PB, Kaur B, Ott MC (2020) Prophylactic negative pressure wound therapy for closed laparotomy incisions: a meta-analysis of randomized controlled trials. Ann Surg 271:67–74. https://doi.org/10.1097/SLA.0000000000003435
Patmo AS, Krijnen P, Tuinebreijer WE, Breederveld RS (2014) The effect of vacuum-assisted closure on the bacterial load and type of bacteria: a systematic review. Adv Wound Care (New Rochelle) 3:383–389. https://doi.org/10.1089/wound.2013.0510
Funding
Funded by grant project of Ministry of Health Care of Slovak Republic No. 2018/16-UKMT-12.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AF, JV, MA, MŽ, MD, MG, and AŠ. The first draft of the manuscript was written by AF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
Board Name: Jessenius Faculty of Medicine Comenius University Bratislava in Martin, IRB Number IRB00005636, Approval Number EK1/2019.
Consent to participate
All patient included in the study signed informed consent.
Consent for publication
All co-authors agreed with the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferko, A., Váňa, J., Adámik, M. et al. Mucosa plication reinforced colorectal anastomosis and trans-anal vacuum drainage: a pilot study with preliminary results. Updates Surg 73, 2145–2154 (2021). https://doi.org/10.1007/s13304-021-01105-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-021-01105-4