Abstract
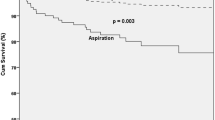
Aspiration detected in the fiberoptic endoscopy evaluation of swallowing (FEES) has been inconsistently associated with pneumonia, with no evidence of the risk of pneumonia from other alterations in swallowing safety detected in FEES. We conducted a dynamic, ambidirectional cohort study involving 148 subjects at risk of dysphagia in a tertiary university hospital. Our aim was to determine the risk of pneumonia attributed to alterations in swallowing safety detected during FEES. We used multivariate negative binomial regression models to adjust for potential confounders. The incidence density rate (IR) of pneumonia in patients with tracheal aspiration of any consistency was 26.6/100 people-years (RR 7.25; 95% CI: 3.50–14.98; P < 0.001). The IR was 19.7/100 people-years (RR 7.85; 95% CI: 3.34–18.47; P < 0.001) in those with laryngeal penetration of any consistency and 18.1/100 people-years (RR 6.24; 95% CI: 2.58–15.09; P < 0.001) in those with pharyngeal residue of any consistency. When adjusted for aspiration, the association of residue and penetration with pneumonia disappeared, suggesting that their risk of pneumonia is dependent on the presence of aspiration and that only aspiration is independently associated with pneumonia. This increased risk of pneumonia was significant in uni- and multivariate negative binomial regression models. We found an independently increased risk of pneumonia among patients with dysphagia and aspiration detected during FEES. Alterations in the oral and pharyngeal phases of swallowing, without aspiration, did not increase the risk of pneumonia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dysphagia is associated with an increased risk of malnutrition, electrolyte imbalance, and pneumonia [1, 2]. Aspiration pneumonia has a mortality rate higher than 20% [3,4,5], generating a high burden on society and increased health-care costs [6, 7]. Fiberoptic endoscopy evaluation of swallowing (FEES) and videofluoroscopic evaluation of swallowing (VFSS) are considered the gold standard tests to evaluate oropharyngeal dysphagia [8, 9].
Both FEES and VFSS findings are related to the efficiency and safety of swallowing. Alterations in swallowing safety include aspiration of food below the vocal cords, penetration of food into the laryngeal vestibule, and persistence of food residue in the pharynx after swallowing [9, 10]. Alterations of swallowing efficiency or function refer to delays in bolus transit from the mouth to the stomach or other alterations in the deglutition function not necessarily compromising swallowing safety [11].
Experts in diagnostic test research advocate a phased approach to evaluating clinical tests, mirroring the 4-phase model used for therapeutic interventions [12, 13]. Such phases include test development, reliability assessment (including inter- and intra-rater reliability), diagnostic accuracy, prognostic capacity for outcomes, and clinical effectiveness in decision-making [12, 13]. Prior research has explored FEES’s reliability [14,15,16] and accuracy [17] as a diagnostic test. Regarding the prognostic capacity of alterations in swallowing safety detected during FEES and VFSS, aspiration detected in VFSS has been associated with a significant increase in the risk of pneumonia [18,19,20]. Aspiration detected in FEES presents an annual risk of pneumonia higher than 20% [21,22,23], potentially increasing the risk by up to three times; however, this evidence is still limited due to the poor control of confounding bias in most studies [24]. Penetration detected in VFSS has also been related to an increased risk of pneumonia [20]. However, the presence of penetration in FEES has not been associated with pneumonia, and the evidence supporting the association between penetration and pneumonia in VFSS is still weak [20, 25, 26]. Similarly, there are few studies on the risk of pneumonia in patients with pharyngeal residue [25, 26].
Therefore, the evidence on the prognostic capacity of FEES findings is scarce or limited by poor control of confounding bias [24], and extrapolating the predictive capacity for dysphagia outcomes from VFSS to FEES would be inappropriate, considering that each diagnostic test requires its own validation and the results of a 2017 meta-analysis comparing FEES and VFSS, which found FEES slightly more sensitive in detecting aspiration, penetration, and residue [17]. Furthermore, it is unclear whether alterations in swallowing safety, other than aspiration, independently increase the risk of pneumonia or if the presence of aspiration is necessary for pneumonia to occur.
Considering the high risk of complications associated with pneumonia in patients with muscular or neurological disorders and other causes of functional oropharyngeal dysphagia combined with the high costs associated with the treatment of this condition, it is crucial to detect high-risk subgroups for pneumonia in order to establish preventive measures [6, 7, 27]. For this reason, we decided to conduct a cohort study to evaluate which of the swallowing alterations, particularly aspiration, penetration, and residue detected in FEES, are associated with an increased risk of pneumonia.
Materials and Methods
We performed an ambidirectional cohort study involving 148 subjects consecutively recruited over a six-year period from an outpatient rehabilitation service at a tertiary care university hospital. The study aimed to explore the relationship between alterations in swallowing safety (penetration, aspiration, or residue) as identified by FEES and the incidence of pneumonia. Subjects with muscular or neurological involvement at risk of functional oropharyngeal dysphagia were included. The exposed cohort was constituted by those subjects with alterations in swallowing safety detected in FEES and the unexposed cohort included subjects without such alterations.
The exclusion criteria were subjects with mechanical dysphagia (tumors or anatomical abnormalities of the upper aero-digestive tract), active cancer, cancer chemotherapy, pregnancy, and asplenia.
Pneumonia was defined as the presence of the symptoms and signs of acute lower respiratory infection (cough, purulent sputum, fever, tachypnea, tachycardia, leukocytosis, or leukopenia), and the presence of infiltrates on a chest radiograph [28, 29].
Socioeconomic level in Colombia is classified according to the place of residence and the payment of public services. Low socioeconomic level is those classified as 1 and 2, medium socioeconomic level to levels 3 and 4, and high socioeconomic level to levels 5 and 6.
Evaluation of Swallowing
All subjects received a clinical evaluation of swallowing that included a search for signs and symptoms of oropharyngeal dysphagia and an assessment of head and neck muscle function. Further details of the clinical evaluation are included in the supplementary appendix. Subjects were considered at risk of oropharyngeal dysphagia when one or more of the following abnormalities were present: symptoms of pharyngeal dysphagia; any compromise of gag reflex; a gastrostomy, jejunostomy, or nasoenteral feeding tube; head or neck muscle compromise; or an orotracheal or tracheostomy tube for more than 14 days. When the clinical evaluation of swallowing was completely normal, a FEES was not performed. Subjects underwent a FEES in case of any abnormality in the clinical evaluation of swallowing or oropharyngeal muscle motor compromise.
The FEES studies were conducted by a pulmonologist with more than 2 years’ experience and 50 completed FEES procedures along with a speech language pathologist. The studies were done with the subjects in a seated position, without anesthetics, and using a flexible fiberoptic endoscope with an outer diameter of 4.1 mm connected to a video system (Olympus LF-GP, Center Valley, PA, USA). The endoscope was lubricated with water-soluble gel, introduced through one of the nostrils, and advanced into the pharynx. The examination included an anatomical and functional exploration of the upper aero-digestive tract, including vocal cord motility. Subsequently, the tip of the endoscope was placed at the velopharynx. A first swallow without food was conducted to evaluate the pharyngeal contraction and the elevation of the larynx. The evaluation of swallowing continued by observing the bolus transport of green-colored foods of different consistencies from the back of the oral cavity to the esophagus and searching for any abnormality in deglutition.
The FEES unit, connected to the video equipment, recorded the procedures. These recordings were then reviewed in real-time and, when necessary, in slow motion after the examination. Further details of the FEES protocol can be found elsewhere [30].
An association with pneumonia was explored for the following alterations of swallowing safety as detected in FEES: residue, penetration, and aspiration. Residue consisted of the persistence of material in the pharynx (pharyngeal walls, valleculae, epiglottis, pyriform sinuses, and base of the tongue) after swallowing, penetration consisted of the entrance of material into the laryngeal vestibule, and aspiration consisted of the passage of material into the trachea below the vocal cords [30]. Given the existing literature validating the intra- and inter-rater reliability [14,15,16] and accuracy [17] of the FEES findings we investigated and our study’s focus on other aspects, such as the prognostic capacity of FEES for predicting the occurrence of pneumonia, we did not include measurements of intra- and inter-rater reliability in our study [14, 15].
Follow-up
The follow-up for the 148 patients spanned five years, comprising two phases. Phase I, the retrospective phase, occurred between the onset of dysphagia (e.g., stroke, brain trauma, or central nervous system infection causing dysphagia) and the FEES. For subjects with a completely normal clinical examination of swallowing, who did not require FEES, this time was between the event causing disability requiring referral to our rehabilitation program and the clinical examination of swallowing. These patients with normal swallowing were included in the unexposed cohort for comparison with the exposed cohort (patients having alterations in swallowing safety detected by FEES). We included this retrospective phase of follow-up in order to analyze the period in which subjects did not receive swallowing rehabilitation, because at the time dysphagia was diagnosed, the subjects were admitted to a standardized protocol aiming to prevent respiratory complications and rehabilitate the swallowing process, which could weak the relationship between dysphagia and pneumonia. Phase II of the follow-up (prospective phase) was between the FEES or the normal clinical examination of swallowing and the end of the study. We determined the frequency of pneumonia during both phases of follow-up. The mean follow-up duration for Phase I was 1.8 years, and for Phase II was 3.7 years, resulting in a total mean follow-up of 5.5 years. Subjects underwent follow-up FEES when indicated by changes in the clinical evaluation of swallowing.
Ethical Considerations
The study was performed according to the international standards of the Helsinki Declaration and national regulations. It was approved by the local Institutional Review Board and all subjects provided informed consent to participate in the study.
Statistical Analysis
Qualitative variables were summarized as frequencies and percentages, and quantitative variables were summarized as means ± standard deviation (SD) if normally distributed, or median and interquartile range (25th–75th percentile) if non-normally distributed. The number of pneumonia episodes were analyzed in terms of incidence density rate (IR), namely the number of pneumonia episodes over the sum of the person-time of the people at risk (number of cases/100 people-years).
We implemented strategies to reduce selection bias, to control observer bias, and to prevent information loss, all of which are detailed in the supplementary appendix.
The independent variables included penetration, aspiration, and pharyngeal residue; the dependent variable was the number of episodes of pneumonia. To control confusion bias, we built multivariate logistic and negative binomial regression models that included the alterations of swallowing safety and the variables that could affect the association between dysphagia and pneumonia selected by biological plausibility and the drawing of directed acyclic graphs (DAG) (Fig. 1). The variables for the bivariate analysis selected by DAG were age at time of event, sex [31], smoking [32], chronic respiratory diseases (COPD, asthma, and other respiratory diseases) [33, 34], antacids [35], proton-pump inhibitors, oral hygiene frequency per day [36, 37], use of inhaled and systemic corticosteroids [38], diabetes, congestive heart failure, use of atypical and typical antipsychotics [39], high alcohol consumption defined as more than 3 drinks per day and/or more than 6 drinks per occasion in women or more than 8 drinks per occasion in men [40, 41], and immunosuppression [42]. The variables associated with the outcome (pneumonia) and the exposure (alterations of swallowing safety) in the univariate analysis with P < 0.25 at two tails and that fulfilled the criteria to be potential confounders according to DAGs (potential association with the exposure and outcome and not being intermediate variables in the causal path from the exposure to the outcome were entered into the multivariate model, and those with statistical significance in the multivariate model or those that changed the regression coefficient of the exposure under study by more than 10% were retained. Statistical significance was set at P < 0.05 (two-tailed).
Causal pathways between comorbidities and pneumonia. Notes: A: age, sex, malnutrition, alcohol consumption; B: chronic obstructive pulmonary disease (COPD), asthma, diabetes; C: compromised pharyngeal function; D: dysphagia; E: antacids; I: immunosuppression; M: central nervous system depressant drugs, cerebrovascular disease, muscular and neuromuscular diseases, compromised state of consciousness, dementia, central nervous system cancer, head and neck cancer, degenerative neurological diseases; O: medications that decrease saliva production, hypertension; P: gastroesophageal reflux, esophageal dysmotility, upper gastrointestinal tract surgery, congestive heart failure; PN: pneumonia; R: inhaled corticosteroid, systemic corticosteroid; S: oral hygiene, smoking; T: cirrhosis; U: infections in the oropharynx and lacerations in the mouth, throat and esophagus; X: xerostomia
To investigate whether the association between penetration, residue, and the occurrence of pneumonia was independent or mediated by aspiration, we constructed a DAG illustrating the corresponding causal pathways (Fig. 2). Aspiration was the potential mediator variable, and thus, it was incorporated into the multivariate models of penetration and residue to assess whether the associations between penetration, residue, and pneumonia ceased.
In our analysis of the effect of penetration, residue, and aspiration on pneumonia occurrence, we considered all subjects exhibiting the respective alteration, regardless of the presence of aspiration. For example, in the analysis of penetration, we incorporated subjects with both penetration and aspiration, as well as those with penetration alone.
Incorporating the aspiration variable into the multivariate models aimed to assess whether penetration or residue necessitated the presence of aspiration to increase the risk of pneumonia. This involved controlling for aspiration, effectively isolating the effect of penetration or residue (i.e., excluding the influence of aspiration) on pneumonia occurrence. If the association of penetration or residue with pneumonia occurrence were independent of the presence of aspiration, such an association would remain statistically significant even after introducing the aspiration variable into the corresponding models.
In our study, we analyzed aspiration, penetration, and pharyngeal residue as dichotomous variables. This approach allowed us to explore independent causal pathways in the multivariate analysis, which would have been challenging with ordinal scales. Moreover, incorporating ordinal, non-normally distributed variables into multivariable models presents statistical challenges that could interfere with achieving our main objectives.
The sample size calculation was performed with data from a prior retrospective study [43]. A total sample size of 148 subjects was calculated estimating a 20% annual incidence of pneumonia in the exposed cohort, a 4% annual incidence of pneumonia in the unexposed cohort, a 12% mean incidence (cohort exposed and unexposed combined), and a follow-up of 5 years (confidence level: 95%; power: 80%; including 10 predictor variables in the multivariate logistic regression model). The software used was Microsoft Excel 2007, and Stata SE, version 15.
Results
A total of 148 patients were recruited from the comprehensive rehabilitation program at the University of La Sabana clinic and followed up for 5 years. The median age was 51 years with 57% being male. The clinical diagnoses with the highest prevalence were cerebrovascular disease (34%), trauma (18%), and neurodegenerative diseases (13%). The most common comorbidities were COPD (11%), heart failure (7%), and diabetes (7%). Approximately 60% of the population used antacids and 18% received psychiatric treatment, as shown in Table 1. The detailed characteristics of patients with and without aspiration may be found in the supplementary appendix (Table 1 of supplementary appendix).
In the Binomial regression model, the risk of pneumonia was significantly higher when the following FEES findings were present: aspiration with an adjusted IR of 26.6 cases of pneumonia per 100 people-years (P < 0.001), penetration with an adjusted IR of 19.7 cases of pneumonia per 100 people-years (P < 0.001), and residue with an adjusted IR of 18.1 cases of pneumonia per 100 people-years (P < 0.001).
The bivariate analysis (Table 2) was conducted using negative binomial regression, as the variables were overdispersed, meaning that the standard deviation of the response variable was greater than its mean. Significant associations were found with the variables of aspiration, penetration, and residue (P < 0.001), and a significant trend was observed with the use of antiacids.
For the multivariate analysis to control for confounding bias, we selected variables that could be potentially associated with the studied outcome (pneumonia) and the exposure (FEES alterations) based on both clinical relevance and a P < 0.25. The clinical relevance was determined by biological plausibility based on previous studies and DAG (Fig. 1).
The relative risk of developing pneumonia increased by 7.25 times (P < 0.001) in patients who had aspiration, 7.85 (P < 0.001) in those who had penetration, and 6.24 times (P < 0.001) in those who had pharyngeal residue, after adjusting for possible confounding variables (Tables 3, 4, 5).
To explore if the association between penetration and residue with pneumonia was independent or depended on the association of these abnormalities with aspiration, the aspiration variable was introduced into the multivariate models of penetration and residue (see corresponding DAGs in Fig. 2). After doing this, the association between penetration and residue with pneumonia disappeared, however, the association between aspiration and pneumonia persisted, suggesting that the association between penetration and residue with pneumonia is not independent, but rather depends on whether these abnormalities cause aspiration, which would ultimately lead to pneumonia (Tables 6, 7).
Discussion
In this study, performed in a cohort of patients with various causes of functional oropharyngeal dysphagia, representative of many swallowing services of tertiary care centers, we found an increased risk of pneumonia in the presence of aspiration, penetration, or residue with any consistency of food in the FEES. However, only aspiration was independently associated with pneumonia because the association of penetration or residue with pneumonia disappears adjusting with aspiration. In addition, we observed that the frequency of mouth washing tended to be associated to a lower risk of pneumonia, meaning that it could be a protective factor for the development of pneumonia, since it possibly decreases oral colonization as reported by Aiemyen et al. [44].
We found a 7.2 times increased risk of pneumonia in patients with detected aspiration in the FEES, similar to those reported by Holas et al. [18] and Pikus et al. [20] in studies performed by VFSS. Previous studies with FEES found an increased risk of pneumonia without reaching statistical significance, except in the study by Takahashi et al., [45] where they found a significant increase in the risk of pneumonia in patients with aspiration of saliva; however, aspiration of food was not associated with pneumonia in that study. The fact that all of these previous studies consistently showed an annual incidence of pneumonia higher than 20% in subjects with oropharyngeal dysphagia when the incidence of pneumonia reported for the general population is below 1.5% [46, 47], and that pooling these studies’ results in a recent meta-analysis [24] reached statistical significance, suggests that the absence of statistical significance in such original publications was probably related to low statistical power due to small sample sizes, or insufficient numbers of patients without aspiration, and not to a real lack of association between aspiration and pneumonia.
Prior research by Pikus et al. [20] and Gurber et al. [48] found a significant increase in pneumonia risk among patients with penetration observed in VFSS, but they did not investigate the effect of adjusting for aspiration in this association. Our findings indicate that the relationship between penetration and pneumonia could be contingent upon whether penetration leads to aspiration rather than penetration itself causing pneumonia. Conversely, no previous studies have demonstrated a link between pneumonia and pharyngeal residue detected in VFSS or FEES. Nevertheless, evidence suggests an elevated risk of aspiration due to pharyngeal residue in both VFSS [49, 50] and FEES [51,52,53]. Our findings point to that the association between residue and pneumonia disappears when adjusting for aspiration, indicating that the relationship may hinge on whether pharyngeal residue leads to aspiration, mirroring the situation observed with penetration.
This study has the limitations of a cohort study; that is, it is exposed to biases and confounding variables which limit its ability to infer definitive causal relationships. However, the fact that this is one of the largest cohort studies that has been done evaluating the risk of pneumonia associated with alterations of swallowing safety detected in FEES, in terms of number of patients and time of follow-up, and that we analyzed outcome (pneumonia) as IR instead of cumulative incidence, as recommended when a subject may have more than one event (pneumonia) [54], helped to decrease the limitations related to statistical power. Furthermore, we took several measures to reduce biases (see the methods section), which may have helped to obtain more accurate risk estimates.
The diagnosis of pneumonia can be a challenge for any study because the diagnostic criteria have no high specificity or sensitivity [29, 33]. However, the diagnosis based on clinical criteria supported by imaging has been widely accepted for more than 20 years, particularly in the centers where these patients were treated; furthermore, it is consistent with the diagnoses used in other studies on dysphagia complications [20, 29, 33, 55]. In addition, the doctors who made the pneumonia diagnoses were unaware of the study and the hypothesis thereof, which minimized the risk of differential misclassification. The risk of non-differential misclassification cannot be completely ruled out in our study and is common in any study that uses a pneumonia outcome, due to the diagnostic difficulties mentioned above, but this type of bias often results in an underestimation of the relative risk (RR) instead of an overestimation, so it does not invalidate a significant RR [56].
Conclusions
Our study presents evidence about the independent significant association between aspiration detected in FEES and the development of pneumonia. In addition, it showed that alterations in the oral and pharyngeal phases of swallowing, without the presence of aspiration, did not significantly increase the risk of developing pneumonia. Patients in whom aspiration is observed during FEES should receive interventions aimed at reducing the risk of pneumonia, including dietary modifications to avoid oral feeding with the food consistency that caused aspiration, proper oral hygiene, influenza and pneumococcal vaccination, avoidance of medications that can increase the risk of pneumonia, and, when clinically indicated (e.g. aspiration of more than two food consistencies) be fed by an alternative route to the oral one [33, 57].
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124:328–36.
Smithard DG, O’Neill PA, Park C, Morris J, Wyatt R, England R, Martin DF. Complications and outcome after acute stroke: does dysphagia matter? Stroke. 1996;27:1200–4. https://doi.org/10.1161/01.str.27.7.1200.
Croghan JE, Burke EM, Caplan S, Denman S. Pilot study of 12-month outcomes of nursing home patients with aspiration on videofluoroscopy. Dysphagia. 1994;9:141–6.
Ickenstein GW, Stein J, Ambrosi D, Goldstein R, Horn M, Bogdahn U. Predictors of survival after severe dysphagic stroke. J Neurol. 2005;252:1510–6. https://doi.org/10.1007/s00415-005-0906-9.
Low J, Wyles C, Wilkinson T, Sainsbury R. The effect of compliance on clinical outcomes for patients with dysphagia on videofluoroscopy. Dysphagia. 2001;16:123–7. https://doi.org/10.1007/s004550011002.
Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age Ageing. 2008;37:258–64. https://doi.org/10.1093/ageing/afn064.
Wilson RD. Mortality and cost of pneumonia after stroke for different risk groups. J Stroke Cerebrovasc Dis. 2012;21:61–7. https://doi.org/10.1016/j.jstrokecerebrovasdis.2010.05.002.
Kelly AM, Leslie P, Beale T, Payten C, Drinnan MJ. Fibreoptic endoscopic evaluation of swallowing and videofluoroscopy: does examination type influence perception of pharyngeal residue severity? Clin Otolaryngol. 2006;31:425–32. https://doi.org/10.1111/j.1749-4486.2006.01292.x.
Wu CH, Hsiao TY, Chen JC, Chang YC, Lee SY. Evaluation of swallowing safety with fiberoptic endoscope: comparison with videofluoroscopic technique. Laryngoscope. 1997;107:396–401.
Langmore SE, Schatz K, Olson N. Endoscopic and videofluoroscopic evaluations of swallowing and aspiration. Ann Otol Rhinol Laryngol. 1991;100:678–81.
Logemann JA. Mechanisms of normal and abnormal swallowing. In: Flint PW, Haughey BH, Lund V, Niparko JK, Robbins KT, Thomas JR, Lesperance MM, editors. Cummings: otolaryngology: head & neck surgery. 6th ed. Philadelphia: Elsevier Saunders; 2015. p. 1500–6.
Colli A, Fraquelli M, Casazza G, Conte D, Nikolova D, Duca P, Thorlund K, Gluud C. The architecture of diagnostic research: from bench to bedside–research guidelines using liver stiffness as an example. Hepatology. 2014;60:408–18. https://doi.org/10.1002/hep.26948.
Lijmer JG, Leeflang M, Bossuyt PMM. Proposals for a phased evaluation of medical tests. Med Decis Making. 2009;29:E13-21. https://doi.org/10.1177/0272989x09336144.
Leder SB, Acton LM, Lisitano HL, Murray JT. Fiberoptic endoscopic evaluation of swallowing (FEES) with and without blue-dyed food. Dysphagia. 2005;20:157–62. https://doi.org/10.1007/s00455-005-0009-x.
Tohara H, Nakane A, Murata S, Mikushi S, Ouchi Y, Wakasugi Y, Takashima M, Chiba Y, Uematsu H. Inter- and intra-rater reliability in fibroptic endoscopic evaluation of swallowing. J Oral Rehabil. 2010;37:884–91. https://doi.org/10.1111/j.1365-2842.2010.02116.x.
Colodny N. Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (FEES) using the penetration-aspiration scale: a replication study. Dysphagia. 2002;17:308–15. https://doi.org/10.1007/s00455-002-0073-4.
Giraldo-Cadavid LF, Leal-Leaño LR, Leon-Basantes GA, Bastidas AR, Garcia R, Ovalle S, Abondano-Garavito JE. Accuracy of endoscopic and videofluoroscopic evaluations of swallowing for oropharyngeal dysphagia. Laryngoscope. 2017;127:2002–10. https://doi.org/10.1002/lary.26419.
Holas MA, DePippo KL, Reding MJ. Aspiration and relative risk of medical complications following stroke. Arch Neurol. 1994;51:1051–3.
Kidd D, Lawson J, Nesbitt R, MacMahon J. The natural history and clinical consequences of aspiration in acute stroke. QJM. 1995;88:409–13.
Pikus L, Levine MS, Yang YX, Rubesin SE, Katzka DA, Laufer I, Gefter WB. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. AJR Am J Roentgenol. 2003;180:1613–6.
Aviv JE. Prospective, randomized outcome study of endoscopy versus modified barium swallow in patients with dysphagia. Laryngoscope. 2000;110:563–74. https://doi.org/10.1097/00005537-200004000-00008.
Lim SH, Lieu PK, Phua SY, Seshadri R, Venketasubramanian N, Lee SH, Choo PW. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia. 2001;16:1–6. https://doi.org/10.1007/s004550000038.
Masiero S, Pierobon R, Previato C, Gomiero E. Pneumonia in stroke patients with oropharyngeal dysphagia: a six-month follow-up study. Neurol Sci. 2008;29:139–45. https://doi.org/10.1007/s10072-008-0925-2.
Giraldo-Cadavid LF, Bastidas AR, Maldonado-Lancheros J, Gasca-Zuluaga DA, Aguilar-Farias MJ, Bohorquez-Tibavisco L. Pneumonia, mortality, and other outcomes associated with unsafe swallowing detected via fiberoptic endoscopic evaluation of swallowing (FEES) in patients with functional oropharyngeal dysphagia: a systematic review and meta-analysis. Dysphagia. 2022;37:1662–72. https://doi.org/10.1007/s00455-022-10427-3.
Weir K, McMahon S, Barry L, Ware R, Masters IB, Chang AB. Oropharyngeal aspiration and pneumonia in children. Pediatr Pulmonol. 2007;42:1024–31. https://doi.org/10.1002/ppul.20687.
Johnson ER, McKenzie SW, Sievers A. Aspiration pneumonia in stroke. Arch Phys Med Rehabil. 1993;74:973–6.
Rofes L, Arreola V, Almirall J, Cabre M, Campins L, Garcia-Peris P, Speyer R, Clave P. Diagnosis and management of oropharyngeal Dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterol Res Pract. 2011. https://doi.org/10.1155/2011/818979.
Gonzales R, Sande MA. Uncomplicated acute bronchitis. Ann Intern Med. 2000;133:981–91. https://doi.org/10.7326/0003-4819-133-12-200012190-00014.
Fabregas N, Ewig S, Torres A, El-Ebiary M, Ramirez J, de La Bellacasa JP, Bauer T, Cabello H. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax. 1999;54:867–73.
Giraldo-Cadavid LF, Pantoja JA, Forero YJ, Gutiérrez HM, Bastidas AR. Aspiration in the fiberoptic endoscopic evaluation of swallowing associated with an increased risk of mortality in a cohort of patients suspected of oropharyngeal dysphagia. Dysphagia. 2020;35:369–77. https://doi.org/10.1007/s00455-019-10036-7.
Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–45. https://doi.org/10.1212/WNL.0b013e31823152b1.
Almirall J, González CA, Balanzó X, Bolíbar I. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest. 1999;116:375–9. https://doi.org/10.1378/chest.116.2.375.
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27-72. https://doi.org/10.1086/511159.
Farr BM, Woodhead MA, Macfarlane JT, Bartlett CL, McCraken JS, Wadsworth J, Miller DL. Risk factors for community-acquired pneumonia diagnosed by general practitioners in the community. Respir Med. 2000;94:422–7. https://doi.org/10.1053/rmed.1999.0743.
Rodríguez LA, Ruigómez A, Wallander MA, Johansson S. Acid-suppressive drugs and community-acquired pneumonia. Epidemiology. 2009;20:800–6. https://doi.org/10.1097/EDE.0b013e3181b5f27d.
Yoneyama T, Yoshida M, Ohrui T, Mukaiyama H, Okamoto H, Hoshiba K, Ihara S, Yanagisawa S, Ariumi S, Morita T, Mizuno Y, Ohsawa T, Akagawa Y, Hashimoto K, Sasaki H. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc. 2002;50:430–3. https://doi.org/10.1046/j.1532-5415.2002.50106.x.
van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, Schols JM, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12:344–54. https://doi.org/10.1016/j.jamda.2010.12.099.
Joo MJ, Au DH, Fitzgibbon ML, Lee TA. Inhaled corticosteroids and risk of pneumonia in newly diagnosed COPD. Respir Med. 2010;104:246–52. https://doi.org/10.1016/j.rmed.2009.10.002.
Trifirò G, Gambassi G, Sen EF, Caputi AP, Bagnardi V, Brea J, Sturkenboom MC. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med. 2010;152(418–425):w139-440. https://doi.org/10.7326/0003-4819-152-7-201004060-00006.
Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2010;138:1789–95. https://doi.org/10.1017/s0950268810000774.
Hodgson R, Alwyn T, John B, Thom B, Smith A. The FAST alcohol screening test. Alcohol Alcohol. 2002;37:61–6. https://doi.org/10.1093/alcalc/37.1.61.
Koivula I, Sten M, Mäkelä PH. Risk factors for pneumonia in the elderly. Am J Med. 1994;96:313–20. https://doi.org/10.1016/0002-9343(94)90060-4.
Giraldo LF, Miranda X, Barros C, Hernandez M, Martinez N, Leon MX, Rengifo ML, Estrada O, Gomez C, Escobar I. Respiratory complications in a cohort of patients at risk for oropharyngeal dysphagia. Chest. 2008;134: p11004.
Aiemyen N, Pothidee T, Sopapornamorn P, Payukaparp P, Luangnam C, Suksan S, Preechasummakul P, Samnieng P. Chemical oral health care and Aspiration Pneumonia (AP) in elderly patients: a systematic literature review. J Int Dent Med Res. 2020;13:372–8.
Takahashi N, Kikutani T, Tamura F, Groher M, Kuboki T. Videoendoscopic assessment of swallowing function to predict the future incidence of pneumonia of the elderly. J Oral Rehabil. 2012;39:429–37. https://doi.org/10.1111/j.1365-2842.2011.02286.x.
Almirall J, Morato I, Riera F, Verdaguer A, Priu R, Coll P, Vidal J, Murgui L, Valls F, Catalan F, et al. Incidence of community-acquired pneumonia and Chlamydia pneumoniae infection: a prospective multicentre study. Eur Respir J. 1993;6:14–8.
Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Karkola K, Korppi M, Kurki S, Rönnberg PR, Seppä A, Soimakallio S, et al. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am J Epidemiol. 1993;137:977–88. https://doi.org/10.1093/oxfordjournals.aje.a116770.
Gurberg J, Birnbaum R, Daniel SJ. Laryngeal penetration on videofluoroscopic swallowing study is associated with increased pneumonia in children. Int J Pediatr Otorhinolaryngol. 2015;79:1827–30. https://doi.org/10.1016/j.ijporl.2015.08.016.
Horner J, Buoyer FG, Alberts MJ, Helms MJ. Dysphagia following brain-stem stroke. Clinical correlates and outcome. Arch Neurol. 1991;48:1170–3.
Nayak VK, Bhattacharyya N, Kotz T, Shapiro J. Patterns of swallowing failure following medialization in unilateral vocal fold immobility. Laryngoscope. 2002;112:1840–4. https://doi.org/10.1097/00005537-200210000-00025.
Wu CH, Hsiao TY, Ko JY, Hsu MM. Dysphagia after radiotherapy: endoscopic examination of swallowing in patients with nasopharyngeal carcinoma. Ann Otol Rhinol Laryngol. 2000;109:320–5.
Ku PK, Vlantis AC, Leung SF, Lee KY, Cheung DM, Abdullah VJ, van Hasselt A, Tong MC. Laryngopharyngeal sensory deficits and impaired pharyngeal motor function predict aspiration in patients irradiated for nasopharyngeal carcinoma. Laryngoscope. 2010;120:223–8. https://doi.org/10.1002/lary.20701.
Kaye GM, Zorowitz RD, Baredes S. Role of flexible laryngoscopy in evaluating aspiration. Ann Otol Rhinol Laryngol. 1997;106:705–9.
Vandenbroucke JP, Pearce N. Incidence rates in dynamic populations. Int J Epidemiol. 2012;41:1472–9. https://doi.org/10.1093/ije/dys142.
Aviv JE. Prospective, randomized outcome study of endoscopy versus modified barium swallow in patients with dysphagia. Laryngoscope. 2000. https://doi.org/10.1097/00005537-200004000-00008.
Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–52. https://doi.org/10.1016/s0140-6736(02)07451-2.
American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. https://doi.org/10.1164/rccm.200405-644ST.
Acknowledgements
The authors are most thankful for the Universidad de La Sabana. This study was funded by La Universidad de La Sabana, code MEDMSc-73-2023.
Funding
Open Access funding provided by Colombia Consortium. Universidad de La Sabana, Funding code MEDMSc-73–2023. Universidad de La Sabana, School of Medicine, Institutional Review Board Reference Number: 4007/06. The study was performed according to the international standards of the Helsinki Declaration and national regulations. It was approved by the local Institutional Review Board and all subjects provided informed consent to participate in the study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Luis F. Giraldo-Cadavid, Valentina Velasco, Diego Insignares, and Natalia Londoño. Ana M. Galvis and Maria Leonor Rengifo also contributed to material preparation and data collection. Alirio R. Bastidas also contributed to data analysis. The first draft of the manuscript was written by Valentina Velasco, Diego Insignares, and Natalia Londoño. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giraldo-Cadavid, L.F., Insignares, D., Velasco, V. et al. Fiberoptic Endoscopy Evaluation of Swallowing (FEES) Findings Associated with High Pneumonia Risk in a Cohort of Patients at Risk of Dysphagia. Dysphagia (2024). https://doi.org/10.1007/s00455-024-10727-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00455-024-10727-w