Abstract
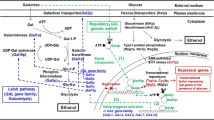
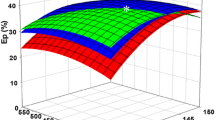
Eucheuma denticulatum is a red macroalgae with a high carbohydrate content. The fermentable sugars from E. denticulatum were obtained through sequential thermal acid hydrolysis, enzymatic saccharification, and detoxification. Thermal acid hydrolysis of E. denticulatum was optimized under the condition of 10% (w/v) slurry content and 300 mM HNO3 at 121 ℃ for 90 min. The maximum monosaccharide concentration after thermal acid hydrolysis was 31.0 g/L with an efficiency (ETAH) of 44.7%. By further enzymatic hydrolysis of pretreated biomass solution under 20 U/mL Cellic CTec2 at 50 ℃ and 160 rpm for 72 h, the maximum monosaccharide concentration reached 79.9 g/L with an efficiency of 66.2% (ES). To remove 5-hydroxymethylfurfural (5-HMF), a fermentation inhibitor, absorption using 2% activated carbon was performed for 2 min. Ethanol fermentation was performed using wild-type and high galactose-adapted strains of Saccharomyces cerevisiae, Kluyveromyces marxianus, and Candida lusitaniae. As a result, galactose-adapted strains showed higher ethanol production than wild-type strains. Especially, the fermentation result by adaptively evolved S. cerevisiae produced the highest ethanol of 37.6 g/L and with YEtOH of 0.48 g/g. Moreover, the transcript level of MIG1 in the galactose-adapted strain was slightly lower than that in the wild-type strain. The application of adaptive evolution of microorganisms was efficient for bioethanol production.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Offei F, Mensah M, Thygesen A, Kemausuor F (2018) Seaweed bioethanol production: a process selection review on hydrolysis and fermentation. Fermentation 4:99. https://doi.org/10.3390/fermentation4040099
Baeyens J, Kang Q, Appels L et al (2015) Challenges and opportunities in improving the production of bio-ethanol. Prog Energy Combust Sci 47:60–88. https://doi.org/10.1016/j.pecs.2014.10.003
Daroch M, Geng S, Wang G (2013) Recent advances in liquid biofuel production from algal feedstocks. Appl Energy 102:1371–1381. https://doi.org/10.1016/j.apenergy.2012.07.031
Naseri A, Jacobsen C, Sejberg JJP et al (2020) Multi-extraction and quality of protein and carrageenan from commercial spinosum (Eucheuma denticulatum). Foods 9:1072. https://doi.org/10.3390/foods9081072
Krstonošić V, Jovičić-Bata J, Maravić N et al (2021) Rheology, structure, and sensory perception of hydrocolloids. Food Struct Funct. https://doi.org/10.1016/B978-0-12-821453-4.00005-3
Kariduraganavar MY, Kittur AA, Kamble RR (2014) Polymer synthesis and processing. Nat Syn Biomed Polym. https://doi.org/10.1016/B978-0-12-396983-5.00001-6
Ra CH, Kim MJ, Jeong GT, Kim SK (2016) Efficient utilization of Eucheuma denticulatum hydrolysates using an activated carbon adsorption process for ethanol production in a 5-L fermentor. Bioprocess Biosyst Eng 40:373–381. https://doi.org/10.1007/S00449-016-1705-7
Nguyen TH, Sunwoo IY, Ra CH et al (2018) Acetone, butanol, and ethanol production from the green seaweed Enteromorpha intestinalis via the separate hydrolysis and fermentation. Bioprocess Biosyst Eng 42:415–424. https://doi.org/10.1007/S00449-018-2045-6
Sunwoo I, Ra C, Kwon S et al (2014) Ethanol production from red, brown and green seaweeds and biosorption of heavy metals by waste seaweed slurry from ethanol production. KSBB J 29:414–420. https://doi.org/10.7841/KSBBJ.2014.29.6.414
Ra CH, Sunwoo IY, Kim S-K (2016) Bioethanol production from macroalgal biomass. J Life Sci 26:976–982. https://doi.org/10.5352/JLS.2016.26.8.976
Kim M-J, Kim J-S, Ra CH, Kim S-K (2013) Bioethanol production from Eucheuma spinosum using various yeasts. KSBB J 28:315–318. https://doi.org/10.7841/KSBBJ.2013.28.5.315
Nguyen TH, Ra CH, Park MR et al (2016) Bioethanol production from seaweed Undaria pinnatifida using various yeasts by separate hydrolysis and fermentation (SHF). Microbiol Biotechnol Lett 44:529–534. https://doi.org/10.4014/MBL.1610.10007
Song B-B, Kim S-K, Jeong G-T (2011) Enzymatic hydrolysis of marine algae Hizikia fusiforme. KSBB J 26:347–351. https://doi.org/10.7841/KSBBJ.2011.26.4.347
Kim SW, Gwak SH, Ra CH, Kim SK (2017) Bioethanol production from the red seaweed Eucheuma denticulatum. Microbiol Biotechnol Lett 45:316–321. https://doi.org/10.4014/MBL.1709.09002
Boopathy R, Bokang H, Daniels L (1993) Biotransformation of furfural and 5-hydroxymethyl furfural by enteric bacteria. J Ind Microbiol 11:147–150. https://doi.org/10.1007/BF01583715
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33. https://doi.org/10.1016/S0960-8524(99)00161-3
Ostergaard S, Roca C, Rønnow B et al (2000) Physiological studies in aerobic batch cultivations of Saccharomyces cerevisiae strains harboring the MEL1 gene. Biotechnol Bioeng 68:252–259. https://doi.org/10.1002/(SICI)1097-0290(20000505)68:3
Yang JW, Park YR, Jeong GT, Kim SK (2021) Bioethanol production from Gracilaria verrucosa using Saccharomyces cerevisiae with adaptive evolution. Microbiol Biotechnol Lett 49:88–94. https://doi.org/10.48022/MBL.2009.09005
Ra CH, Kim YJ, Lee SY et al (2015) Effects of galactose adaptation in yeast for ethanol fermentation from red seaweed, Gracilaria verrucosa. Bioprocess Biosyst Eng 38:1715–1722. https://doi.org/10.1007/S00449-015-1411-X
Kuang MC, Kominek J, Alexander WG et al (2018) Repeated cis-regulatory tuning of a metabolic bottleneck gene during evolution. Mol Biol Evol 35:1968–1981. https://doi.org/10.1093/molbev/msy102
Sirr A, Cromie GA, Jeffery EW et al (2015) Allelic variation, aneuploidy, and nongenetic mechanisms suppress a monogenic trait in yeast. Genetics 199:247–262. https://doi.org/10.1534/genetics.114.170563/-/DC1
Sunwoo IY, Sukwong P, Park YR et al (2020) Enhancement of galactose uptake from Kappaphycus alvarezii using Saccharomyces cerevisiae through deletion of negative regulators of GAL genes. Appl Biochem Biotechnol 193:577–588. https://doi.org/10.1007/S12010-020-03434-3
Nguyen TH, Ra CH, Sunwoo IY et al (2016) Bioethanol production from Gracilaria verrucosa using Saccharomyces cerevisiae adapted to NaCl or galactose. Bioprocess Biosyst Eng 40:529–536. https://doi.org/10.1007/S00449-016-1718-2
Sukwong P, Ra CH, Sunwoo IY et al (2018) Improved fermentation performance to produce bioethanol from Gelidium amansii using Pichia stipitis adapted to galactose. Bioprocess Biosyst Eng 41:953–960. https://doi.org/10.1007/S00449-018-1926-Z
Park Y, Sunwoo IY, Yang J et al (2020) Comparison of ethanol yield coefficients using Saccharomyces cerevisiae, Candida lusitaniae, and Kluyveromyces marxianus adapted to high concentrations of galactose with Gracilaria verrucosa as substrate. J Microbiol Biotechnol 30:930–936. https://doi.org/10.4014/jmb.2002.02014
Sunwoo IY, Kwon JE, Jeong GT, Kim SK (2019) Optimization of hyper-thermal acid hydrolysis and enzymatic saccharification of Ascophyllum nodosum for ethanol production with mannitol-adapted yeasts. Bioprocess Biosyst Eng 42:1255–1262. https://doi.org/10.1007/S00449-019-02123-8
Sukwong P, Sunwoo IY, Jeong DY et al (2020) Improvement of bioethanol production by Saccharomyces cerevisiae through the deletion of GLK1, MIG1 and MIG2 and overexpression of PGM2 using the red seaweed Gracilaria verrucosa. Process Biochem 89:134–145. https://doi.org/10.1016/J.PROCBIO.2019.10.030
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Saha BC, Iten LB, Cotta MA, Wu YV (2005) Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem 40:3693–3700. https://doi.org/10.1016/j.procbio.2005.04.006
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26. https://doi.org/10.1007/S00253-004-1642-2/figures/3
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74:17–24. https://doi.org/10.1016/S0960-8524(99)00160-1
Li M, Li W, Lu Y et al (2017) High conversion of glucose to 5-hydroxymethylfurfural using hydrochloric acid as a catalyst and sodium chloride as a promoter in a water/γ-valerolactone system. RSC Adv 7:14330–14336. https://doi.org/10.1039/c7ra00701a
Kim SK, Park DH, Song SH et al (2013) Effect of fermentation inhibitors in the presence and absence of activated charcoal on the growth of Saccharomyces cerevisiae. Bioprocess Biosyst Eng 36:659–666. https://doi.org/10.1007/S00449-013-0888-4/figures/5
Nguyen TH, Sunwoo IY, Jeong GT, Kim SK (2019) Detoxification of hydrolysates of the red seaweed Gelidium amansii for improved bioethanol production. Appl Biochem Biotechnol 188:977–990. https://doi.org/10.1007/S12010-019-02970-X
Sukwong P, Sunwoo IY, Nguyen TH et al (2019) R-phycoerythrin, R-phycocyanin and ABE production from Gelidium amansii by Clostridium acetobutylicum. Process Biochem 81:139–147. https://doi.org/10.1016/j.procbio.2019.03.023
Cho Y, Kim M-J, Kim S-K (2013) Ethanol production from seaweed, Enteromorpha intestinalis, by separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SSF) with Saccharomyces cerevisiae. KSBB J 28:366–371. https://doi.org/10.7841/ksbbj.2013.28.6.366
Ra CH, Nguyen TH, Jeong GT, Kim SK (2016) Evaluation of hyper thermal acid hydrolysis of Kappaphycus alvarezii for enhanced bioethanol production. Bioresour Technol 209:66–72. https://doi.org/10.1016/j.biortech.2016.02.106
Ahn DJ, Kim SK, Yun HS (2012) Optimization of pretreatment and saccharification for the production of bioethanol from water hyacinth by Saccharomyces cerevisiae. Bioprocess Biosyst Eng 35:35–41. https://doi.org/10.1007/S00449-011-0600-5/figures/5
Sukwong P, Sunwoo IY, Jeong DY et al (2019) Enhancement of bioethanol production from Gracilaria verrucosa by Saccharomyces cerevisiae through the overexpression of SNR84 and PGM2. Bioprocess Biosyst Eng 42:1421–1433. https://doi.org/10.1007/S00449-019-02139-0
Wu CH, Chien WC, Chou HK et al (2014) Sulfuric acid hydrolysis and detoxification of red alga Pterocladiella capillacea for bioethanol fermentation with thermotolerant yeast Kluyveromyces marxianus. J Microbiol Biotechnol 24:1245–1253. https://doi.org/10.4014/jmb.1402.02038
Park JH, Kim SH, Park HD et al (2014) Simultaneous utilization of galactose and glucose by Saccharomyces cerevisiae mutant strain for ethanol production. Renew Energy 65:213–218. https://doi.org/10.1016/j.renene.2013.09.010
Sunwoo IY, Ra CH, Jeong GT, Kim SK (2016) Evaluation of ethanol production and bioadsorption of heavy metals by various red seaweeds. Bioprocess Biosyst Eng 39:915–923. https://doi.org/10.1007/S00449-016-1571-3
Nguyen TH, Ra CH, Sunwoo IY et al (2016) Evaluation of galactose adapted yeasts for bioethanol fermentation from Kappaphycus alvarezii hydrolyzates. J Microbiol Biotechnol 26:1259–1266. https://doi.org/10.4014/JMB.1602.02019
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2019R1F1A1041288). Partially, this work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2022R1F1A1074224).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, J., Sunwoo, I., Jo, H. et al. Enhancement of galactose uptake for bioethanol production from Eucheuma denticulatum hydrolysate using galactose-adapted yeasts. Bioprocess Biosyst Eng 46, 839–850 (2023). https://doi.org/10.1007/s00449-023-02868-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02868-3