Abstract
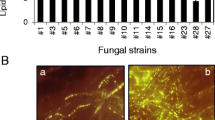
This study evaluated the bioherbicidal potential of wild fungi grown on microalgal biomass from the digestate treatment of biogas production. Four fungal isolates were used and the extracts were evaluated for the activity of different enzymes and characterized by gas chromatography coupled with mass spectrometry. The bioherbicidal activity was assessed by application on Cucumis sativus, and the leaf damage was visually estimated. The microorganisms showed potential as agents producing an enzyme pool. The obtained fungal extracts presented different organic compounds, most acids, and when applied to Cucumis sativus, showed high levels of leaf damage (80–100 ± 3.00%, deviation relative to the observed average damage). Therefore, the microbial strains are potential biological control agents of weeds, which, together with the microalgae biomass, offer the appropriate conditions to obtain an enzyme pool of biotechnological relevance and with favorable characteristics to be explored as bioherbicides, addressing aspects within the environmental sustainability.
Graphical abstract


Similar content being viewed by others
Data availability
The datasets generated for this study are available on request to the corresponding author.
References
Macías-Rubalcava ML, Garrido-Santos MY (2022) Phytotoxic compounds from endophytic fungi. Appl Microbiol Biotechnol 106:931–950. https://doi.org/10.1007/s00253-022-11773-w
Perotti VE, Larran AS, Palmieri VE et al (2020) Herbicide resistant weeds: a call to integrate conventional agricultural practices, molecular biology knowledge and new technologies. Plant Sci. https://doi.org/10.1016/j.plantsci.2019.110255
Verma D, Banjo T, Chawan M, et al (2020) Microbial Control of Pests and Weeds. In: Natural Remedies for Pest, Disease and Weed Control. Elsevier
FAO (2022) Sustainable Development Goals. https://www.fao.org/sustainable-development-goals/goals/goal-2/en/. Accessed 12 Apr 2022
Cordeau S, Triolet M, Wayman S et al (2016) Bioherbicides: Dead in the water? a review of the existing products for integrated weed management. Crop Prot 87:44–49. https://doi.org/10.1016/j.cropro.2016.04.016
Radhakrishnan R, Alqarawi AA, AbdAllah EF (2018) Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol Environ Saf 158:131–138. https://doi.org/10.1016/j.ecoenv.2018.04.018
Hasan M, Ahmad-Hamdani MS, Rosli AM, Hamdan H (2021) Bioherbicides: an eco-friendly tool for sustainable weed management. Plants 10:1212. https://doi.org/10.3390/plants10061212
Bordin ER, Frumi Camargo A, Rossetto V et al (2018) Non-Toxic bioherbicides obtained from trichoderma koningiopsis can be applied to the control of weeds in agriculture crops. Ind Biotechnol 14:157–163. https://doi.org/10.1089/ind.2018.0007
Adetunji CO, Oloke JK, Bello OM et al (2019) Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ Technol Innov 13:304–317. https://doi.org/10.1016/j.eti.2018.12.006
Aita BC, Spannemberg SS, Schmaltz S et al (2019) Production of cell-wall degrading enzymes by solid-state fermentation using agroindustrial residues as substrates. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2019.103193
Reichert Júnior FW, Scariot MA, Forte CT et al (2019) New perspectives for weeds control using autochthonous fungi with selective bioherbicide potential. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e01676
Camargo AF, Venturin B, Bordin ER et al (2020) A low-genotoxicity bioherbicide obtained from trichoderma koningiopsis fermentation in a stirred-tank bioreactor. Ind Biotechnol 16:176–181. https://doi.org/10.1089/ind.2019.0024
Costa JAV, Freitas BCB, Cruz CG et al (2019) Potential of microalgae as biopesticides to contribute to sustainable agriculture and environmental development. J Environ Sci Health B 54:366–375. https://doi.org/10.1080/03601234.2019.1571366
Michelon W, da Silva MLB, Matthiensen A et al (2022) Amino acids, fatty acids, and peptides in microalgae biomass harvested from phycoremediation of swine wastewaters. Biomass Convers Biorefin 12:869–880. https://doi.org/10.1007/s13399-020-01263-2
Michelon W, Da Silva MLB, Mezzari MP et al (2016) Effects of nitrogen and phosphorus on biochemical composition of microalgae polyculture harvested from phycoremediation of piggery wastewater digestate. Appl Biochem Biotechnol 178:1407–1419. https://doi.org/10.1007/s12010-015-1955-x
Stefanski FS, Camargo AF, Scapini T, et al (2020) Potential Use of Biological Herbicides in a Circular Economy Context: A Sustainable Approach. Front Sustain Food Syst 4:. https://doi.org/10.3389/fsufs.2020.521102
Camargo AF, Stefanski FS, Scapini T et al (2019) Resistant weeds were controlled by the combined use of herbicides and bioherbicides. Environ Qual Manage 29:37–42. https://doi.org/10.1002/tqem.21643
Cadete RM, Melo-Cheab MA, Dussán KJ et al (2017) Production of bioethanol in sugarcane bagasse hemicellulosic hydrolysate by Scheffersomyces parashehatae, scheffersomyces illinoinensis and Spathaspora arborariae isolated from Brazilian ecosystems. J Appl Microbiol 123:1203–1213. https://doi.org/10.1111/jam.13559
Barretto DA, Avchar R, Carvalho C et al (2018) Blastobotrys bombycis sp. nov., a d-xylose-fermenting yeast isolated from the gut of the silkworm larva Bombyx mori. Int J Syst Evol Microbiol 68:2638–2643. https://doi.org/10.1099/ijsem.0.002890
Schmitz A, Riesner D (2006) Purification of nucleic acids by selective precipitation with polyethylene glycol 6000. Anal Biochem 354:311–313. https://doi.org/10.1016/j.ab.2006.03.014
Fuwa H (1954) A new method for microdetermination of amylase activity by the use of amylose as the substrate. J Biochem 41:583–603. https://doi.org/10.1093/oxfordjournals.jbchem.a126476
Pongsawadi P, Yagisawa M (1987) Screening and identification of a cyclomaltoxtrinv glucanotransferase-producing bacteria. J Ferment Technol 65:463–467. https://doi.org/10.1016/0385-6380(87)90144-0
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268. https://doi.org/10.1351/pac198759020257
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Hou H, Zhou J, Wang J et al (2004) Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem 39:1415–1419. https://doi.org/10.1016/S0032-9592(03)00267-X
Treichel H, Sbardelotto M, Venturin B et al (2017) Lipase production from a newly isolated aspergillus niger by solid state fermentation using canola cake as substrate. Curr Biotechnol. https://doi.org/10.2174/2211550105666151124193225
Khan AA, Robinson DS (1994) Hydrogen donor specificity of mango isoperoxidases. Food Chem 49:407–410. https://doi.org/10.1016/0308-8146(94)90013-2
Devaiah SP, Shetty HS (2009) Purification of an infection-related acidic peroxidase from pearl millet seedlings. Pestic Biochem Physiol 94:119–126. https://doi.org/10.1016/j.pestbp.2009.04.010
Todero I, Confortin TC, Luft L et al (2020) Concentration of exopolysaccharides produced by Fusarium fujikuroi and application of bioproduct as an effective bioherbicide. Environ Technol 41:2742–2749. https://doi.org/10.1080/09593330.2019.1580775
Brazilian Society of Weed Science (1995) Procedures for Installation, Evaluation and Analysis of Experiments with Herbicides
Lerin et al (2010) Microorganisms screening for limonene oxidation. Ciênc Tecnol Aliment 30:399–405
Kamoun O, Muralitharan G, Belghith H et al (2019) Suitable carbon sources selection and ranking for biodiesel production by oleaginous Mucor circinelloides using multi-criteria analysis approach. Fuel 257:1–13. https://doi.org/10.1016/j.fuel.2019.116117
Rodrigues P, De O, Gurgel LVA, Pasquini D et al (2020) Lignocellulose-degrading enzymes production by solid-state fermentation through fungal consortium among Ascomycetes and Basidiomycetes. Renew Energy 145:2683–2693. https://doi.org/10.1016/j.renene.2019.08.041
Preczeski KP, Dalastra C, Czapela FF et al (2020) Fusarium oxysporum and aspergillus sp. as keratinase producers using swine hair from agroindustrial residues. Front Bioeng Biotechnol 8:1–8. https://doi.org/10.3389/fbioe.2020.00071
da Rosa-Garzon NG, Laure HJ, Rosa JC, Cabral H (2019) Fusarium oxysporum cultured with complex nitrogen sources can degrade agricultural residues: evidence from analysis of secreted enzymes and intracellular proteome. Renew Energy 133:941–950. https://doi.org/10.1016/j.renene.2018.10.100
de Oliveira BH, Coradi GV, de Oliva-Netodo Nascimento VMG, P (2020) Biocatalytic benefits of immobilized Fusarium sp. (GFC) lipase from solid state fermentation on free lipase from submerged fermentation. Ind Crops Prod 147:1–10. https://doi.org/10.1016/j.indcrop.2020.112235
Amoah J, Ho S-H, Hama S et al (2016) Converting oils high in phospholipids to biodiesel using immobilized Aspergillus oryzae whole-cell biocatalysts expressing Fusarium heterosporum lipase. Biochem Eng J 105:10–15. https://doi.org/10.1016/j.bej.2015.08.007
Pessôa MG, Paulino BN, Mano MCR et al (2017) Fusarium species—a promising tool box for industrial biotechnology. Appl Microbiol Biotechnol 101:3493–3511. https://doi.org/10.1007/s00253-017-8255-z
Rodríguez RD, Heredia G, Siles JA et al (2019) Enhancing laccase production by white-rot fungus Funalia floccosa LPSC 232 in co-culture with Penicillium commune GHAIE86. Folia Microbiol (Praha) 64:91–99. https://doi.org/10.1007/s12223-018-0635-y
Li W, Yu J, Li Z, Yin W-B (2019) Rational design for fungal laccase production in the model host Aspergillus nidulans. Sci China Life Sci 62:84–94. https://doi.org/10.1007/s11427-017-9304-8
Kumar AN, Chatterjee S, Hemalatha M et al (2020) Deoiled algal biomass derived renewable sugars for bioethanol and biopolymer production in biorefinery framework. Bioresour Technol 296:1–7. https://doi.org/10.1016/j.biortech.2019.122315
Llamas M, Magdalena JA, Tomás-Pejó E, González-Fernández C (2020) Microalgae-based anaerobic fermentation as a promising technology for producing biogas and microbial oils. Energy 206:1–8. https://doi.org/10.1016/j.energy.2020.118184
Chu R, Li S, Zhu L et al (2021) A review on co-cultivation of microalgae with filamentous fungi: efficient harvesting, wastewater treatment and biofuel production. Renew Sustain Energy Rev 139:1–17. https://doi.org/10.1016/j.rser.2020.110689
Alamsjah MA, Hirao S, Ishibashi F et al (2008) Algicidal activity of polyunsaturated fatty acids derived from Ulva fasciata and U. pertusa (Ulvaceae, Chlorophyta) on phytoplankton. J Appl Phycol 20:713–720. https://doi.org/10.1007/s10811-007-9257-5
Wu J-T, Chiang Y-R, Huang W-Y, Jane W-N (2006) Cytotoxic effects of free fatty acids on phytoplankton algae and cyanobacteria. Aquat Toxicol 80:338–345. https://doi.org/10.1016/j.aquatox.2006.09.011
Li ZR, Amist N, Bai LY (2019) Allelopathy in sustainable weeds management. Allelopath J 48:109–138
Pardo-Muras M, Puig CG, López-Nogueira A et al (2018) On the bioherbicide potential of Ulex europaeus and Cytisus scoparius: profiles of volatile organic compounds and their phytotoxic effects. PLoS ONE. https://doi.org/10.1371/journal.pone.0205997
Norsworthy JK, Meehan JT (2005) Use of isothiocyanates for suppression of palmer amaranth (Amaranthus palmeri ), pitted morningglory (Ipomoea lacunosa), and yellow nutsedge (Cyperus esculentus). Weed Sci 53:884–890. https://doi.org/10.1614/WS-05-056R.1
Khanh TD, Chung IM, Tawata S, Xuan TD (2006) Weed suppression by Passiflora edulis and its potential allelochemicals. Weed Res 46:296–303. https://doi.org/10.1111/j.1365-3180.2006.00512.x
Ulloa-Benítez Á, Medina-Romero YM, Sánchez-Fernández RE et al (2016) Phytotoxic and antimicrobial activity of volatile and semi-volatile organic compounds from the endophyte Hypoxylon anthochroum strain Blaci isolated from Bursera lancifolia (Burseraceae). J Appl Microbiol 121:380–400. https://doi.org/10.1111/jam.13174
Triolet M, Guillemin J, Andre O, Steinberg C (2020) Fungal-based bioherbicides for weed control: a myth or a reality? Weed Res 60:60–77. https://doi.org/10.1111/wre.12389
Michalak I, Chojnacka K (2015) Algae as production systems of bioactive compounds. Eng Life Sci 15:160–176. https://doi.org/10.1002/elsc.201400191
Tubeileh AM, Souikane RT (2020) Effect of olive vegetation water and compost extracts on seed germination of four weed species. Curr Plant Biol 22:1–6. https://doi.org/10.1016/j.cpb.2020.100150
Mutale-joan C, Redouane B, Najib E et al (2020) Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci Rep 10:2820. https://doi.org/10.1038/s41598-020-59840-4
Mzibra A, Aasfar A, Benhima R et al (2021) Biostimulants derived from moroccan seaweeds: seed germination metabolomics and Growth promotion of tomato plant. J Plant Growth Regul 40:353–370. https://doi.org/10.1007/s00344-020-10104-5
Acknowledgements
The authors thank the Brazilian Funding Agencies: Brazilian National Council for Scientific and Technological Development (CNPq), Coordination of the Superior Level Staff Improvement (CAPES), the support of the Bioprocess and Biotechnology for Food Research Center (Biofood), which is funded through the Research Support Foundation of Rio Grande do Sul (FAPERGS) (FAPERGS-22/2551-0000397-4), Federal University of Fronteira Sul (UFFS) and Federal University of Santa Catarina (UFSC) for the financial support.
Funding
CAPES, CNPq and FAPERGS.
Author information
Authors and Affiliations
Contributions
AFC, GF, and HT conceived and designed the study. AFC analyzed the data. AFC and CB drafted the manuscript. CD, AU, TS, CB, NK, WM, and LL helped in the experimental part and carefully revised the manuscript. SLAJ, MAT, and OB helped isolate strains from the intestines of caterpillars. SLAJ, AJM, MAT, OB, SRP, GF, HT critically reviewed and supervised the development of the paper. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors agreed with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Camargo, A.F., Dalastra, C., Ulrich, A. et al. The bioherbicidal potential of isolated fungi cultivated in microalgal biomass. Bioprocess Biosyst Eng 46, 665–679 (2023). https://doi.org/10.1007/s00449-023-02852-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02852-x