Abstract
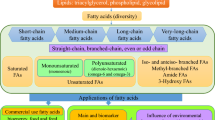
Algae-based wastewater tertiary treatment systems have been drawing attention to eco-friendly companies due to high remediation effectiveness and production of valuable raw material. The amino acids, fatty acids, and peptides from microalgae harvested from a pilot-scale phycoremediation system treating swine wastewater were determined. The maximum microalgae concentration of 247 ± 3.4 mg L−1 was obtained after 11 days when phosphate and ammonium were completely removed. The AA content showed relatively high concentrations (as % of total protein) of essential amino acids such as leucine (4.1), lysine (2.5), phenylalanine (2.6), and threonine (2.4). The fatty acid profile was composed of 5.3% polyunsaturated (as C18:2 and C18:3) and ~ 10% of unsaturated (mainly C16:1 and C18:1). About 25 bioactive peptides related to antioxidative, anti-inflammatory, and anticarcinogenic properties were found. Therefore, microalgae biomass produced during phycoremediation of swine wastewaters seems promising as a source of alternative feedstock with high-added value molecules.

Similar content being viewed by others
References
Yarnold J, Karan H, Oey M, Hankamer B (2019) Microalgal aquafeeds as part of a circular bioeconomy. Trends Plant Sci 24:959–970. https://doi.org/10.1016/j.tplants.2019.06.005
ABPA (2019) ABPA - Associação Brasileira de Proteína Animal – Relatório anual (2018) São Paulo: Disposable at:http://abpa-br.com.br/storage/files/relatorio-anual-2018.pdf
Veroneze ML, Schwantes D, Gonçalves AC et al (2019) Production of biogas and biofertilizer using anaerobic reactors with swine manure and glycerin doses. J Clean Prod 213:176–184. https://doi.org/10.1016/j.jclepro.2018.12.181
Singh J, Dhar DW (2019) Overview of carbon capture technology: microalgal biorefinery concept and state-of-the-art. Front Mar Sci 6:. https://doi.org/10.3389/fmars.2019.00029
Sharma J, Kumar V, Kumar SS et al (2020) Microalgal consortia for municipal wastewater treatment–lipid augmentation and fatty acid profiling for biodiesel production. J Photochem Photobiol B Biol 202. https://doi.org/10.1016/j.jphotobiol.2019.111638
Jacob-Lopes E, Maroneze MM, Deprá MC et al (2019) Bioactive food compounds from microalgae: an innovative framework on industrial biorefineries. Curr Opin Food Sci 25:1–7. https://doi.org/10.1016/j.cofs.2018.12.003
Madeira MS, Cardoso C, Lopes PA et al (2017) Microalgae as feed ingredients for livestock production and meat quality: a review. Livest Sci 205:111–121. https://doi.org/10.1016/j.livsci.2017.09.020
Costa JAV, Moreira JB, Fanka LS, et al (2020) Microalgal biotechnology applied in biomedicine. In: Handbook of algal science, technology and medicine. Elsevier, pp 429–439
Lu W, Liu S, Lin Z, Lin M (2020) Enhanced microalgae growth for biodiesel production and nutrients removal in raw swine wastewater by carbon sources supplementation. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-020-01135-w
Canizares-Villanueva RO, Dominguez AR, Cruz MS, Rios-Leal E (1995) Chemical composition of cyanobacteria grown in diluted, aerated swine wastewater. 51:111–116
Moheimani NR, Vadiveloo A, Ayre JM, Pluske JR (2018) Nutritional profile and in vitro digestibility of microalgae grown in anaerobically digested piggery effluent. Algal Res 35:362–369. https://doi.org/10.1016/j.algal.2018.09.007
Michelon W, Da Silva MLB, Mezzari MP et al (2015) Effects of nitrogen and phosphorus on biochemical composition of microalgae polyculture harvested from phycoremediation of piggerywastewater digestate. Appl Biochem Biotechnol 178:1407–1419. https://doi.org/10.1007/s12010-015-1955-x
Chu F-F, Chu P-N, Cai P-J et al (2013) Phosphorus plays an important role in enhancing biodiesel productivity of Chlorella vulgaris under nitrogen deficiency. Bioresour Technol 134:341–346. https://doi.org/10.1016/j.biortech.2013.01.131
AOAC (2000) Official Methods of Analysis of AOAC International, 15th Ed. Association of Official Analytical Chemists, Washington, DC
AOCS (2013) In: Firestone D (ed) Official Methods and Recommended Practices of the AOCS, (6th Ed.). American Oil Chemists’ Society, Champaign
Zhang X, Petruzziello F, Zani F et al (2012) High identification rates of endogenous neuropeptides from mouse brain. J Proteome Res 11:2819–2827. https://doi.org/10.1021/pr3001699
Minkiewicz P, Dziuba J, Iwaniak A et al (2008) BIOPEP database and other programs for processing bioactive peptide sequences. J AOAC Int 91:965–980
Mezzari MP, da Silva MLB, Nicoloso RS et al (2013) Assessment of N2O emission from a photobioreactor treating ammonia-rich swine wastewater digestate. Bioresour Technol 149:327–332. https://doi.org/10.1016/j.biortech.2013.09.065
Nagarajan D, Kusmayadi A, Yen H-W et al (2019) Current advances in biological swine wastewater treatment using microalgae-based processes. Bioresour Technol 289:121718. https://doi.org/10.1016/j.biortech.2019.121718
Cheng DL, Ngo HH, Guo WS et al (2019) Microalgae biomass from swine wastewater and its conversion to bioenergy. Bioresour Technol 275:109–122. https://doi.org/10.1016/j.biortech.2018.12.019
Sui Y, Muys M, Van de Waal DB et al (2019) Enhancement of co-production of nutritional protein and carotenoids in Dunaliella salina using a two-phase cultivation assisted by nitrogen level and light intensity. Bioresour Technol 287:121398. https://doi.org/10.1016/j.biortech.2019.121398
Chinnasamy S, Bhatnagar A, Claxton R, Das KC (2010) Biomass and bioenergy production potential of microalgae consortium in open and closed bioreactors using untreated carpet industry effluent as growth medium. Bioresour Technol 101:6751–6760. https://doi.org/10.1016/j.biortech.2010.03.094
Zhu L, Wang Z, Shu Q et al (2013) Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res 47:4294–4302. https://doi.org/10.1016/j.watres.2013.05.004
Converti A, Casazza AA, Ortiz EY et al (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48:1146–1151. https://doi.org/10.1016/j.cep.2009.03.006
Zhang L, Wang N, Yang M et al (2019) Lipid accumulation and biodiesel quality of Chlorella pyrenoidosa under oxidative stress induced by nutrient regimes. Renew Energy 143:1782–1790. https://doi.org/10.1016/j.renene.2019.05.081
Koyande AK, Chew KW, Rambabu K et al (2019) Microalgae: a potential alternative to health supplementation for humans. Food Sci Human Wellness 8:16–24. https://doi.org/10.1016/j.fshw.2019.03.001
FAO/WHO (1973) Report of a Joint FAO/WHO Expert Committee. In: Food and Agriculture Organization (ed) Energy and Protein Requirements
Rostagno HS, Albino LFT, Donzele JL, et al (2017) Tabelas Brasileiras para Aves e Suínos: Composição de alimentos e exigências nutricionais., 4th ed.
Xupeng C, Song X, Xuran F (2017) Amino acid changes during energy storage compounds accumulation of microalgae under the nitrogen depletion. In: Amino acid: new insights and roles in plant and animal. pp 197–208
Sun Y, Teng T, Bai G et al (2020) Protein-restricted diet balanced for lysine, methionine, threonine, and tryptophan for nursery pigs elicits subsequent compensatory growth and has long term effects on protein metabolism and organ development. Anim Feed Sci Technol 270:114712. https://doi.org/10.1016/j.anifeedsci.2020.114712
Yang Z, Liao SF (2019) Physiological effects of dietary amino acids on gut health and functions of swine. Front Vet Sci 6:. https://doi.org/10.3389/fvets.2019.00169
Song CH, Seung Min O, Lee S et al (2020) The ratio of dietary n-3 polyunsaturated fatty acids influences the fat composition and lipogenic enzyme activity in adipose tissue of growing pigs. Food Sci Anim Resour 40:242–253. https://doi.org/10.5851/kosfa.2020.e8
Rossi R, Pastorelli G, Cannata S, Corino C (2010) Recent advances in the use of fatty acids as supplements in pig diets: a review. Anim Feed Sci Technol 162:1–11. https://doi.org/10.1016/j.anifeedsci.2010.08.013
Sun HY, Yun HM, Kim IH (2020) Effects of dietary n -6/ n -3 polyunsaturated fatty acids ratio on growth performance, apparent digestibility, blood lipid profiles, fecal microbiota, and meat quality in finishing pigs. Can J Anim Sci 100:272–281. https://doi.org/10.1139/cjas-2019-0072
Katiyar R, Arora A (2020) Health promoting functional lipids from microalgae pool: a review. Algal Res 46:101800. https://doi.org/10.1016/j.algal.2020.101800
Posser CJM, Almeida LM, Moreira F et al (2018) Supplementation of diets with omega-3 fatty acids from microalgae: effects on sow reproductive performance and metabolic parameters. Livest Sci 207:59–62. https://doi.org/10.1016/j.livsci.2017.11.006
Sardi L, Martelli G, Lambertini L, et al (2006) Effects of a dietary supplement of DHA-rich marine algae on Italian heavy pig production parameters. 103:95–103. https://doi.org/10.1016/j.livsci.2006.01.009
Roszkos R, Tóth T, Mézes M (2020) Review: practical use of n-3 fatty acids to improve reproduction parameters in the context of modern sow nutrition. Animals 10:1141. https://doi.org/10.3390/ani10071141
Gan X, Huang J, Zhou C et al (2019) Chemosphere relationship between selenium removal efficiency and production of lipid and hydrogen by Chlorella vulgaris. Chemosphere 217:825–832. https://doi.org/10.1016/j.chemosphere.2018.11.075
Norashikin N, Hong S, Aziz A, San T (2018) Metabolic engineering of fatty acid biosynthesis in Chlorella vulgaris using an endogenous omega-3 fatty acid desaturase gene with its promoter. Algal Res 31:262–275. https://doi.org/10.1016/j.algal.2018.02.020
Patel A, Karageorgou D, Rova E et al (2020) An overview of potential oleaginous microorganisms and their role in biodiesel and omega-3 fatty acid-based industries. Microorganisms 8:434. https://doi.org/10.3390/microorganisms8030434
Romanova EV, Sweedler JV (2015) Peptidomics for the discovery and characterization of neuropeptides and hormones. Trends Pharmacol Sci 36:579–586. https://doi.org/10.1016/j.tips.2015.05.009
Selvaraj G, Kaliamurth S, Cakmak ZE, Cakmak T (2017) RuBisCO of microalgae as potential targets for nutraceutical peptides: a computational study. Biotechnology(Faisalabad) 16:130–144. https://doi.org/10.3923/biotech.2017.130.144
Tejano LA, Peralta JP, Yap EES et al (2019) Prediction of bioactive peptides from Chlorella sorokiniana proteins using proteomic techniques in combination with bioinformatics analyses. Int J Mol Sci 20:1786. https://doi.org/10.3390/ijms20071786
Sheih I-C, Wu T-K, Fang TJ (2009) Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour Technol 100:3419–3425. https://doi.org/10.1016/j.biortech.2009.02.014
Sheih I-C, Fang TJ, Wu T-K (2009) Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem 115:279–284. https://doi.org/10.1016/j.foodchem.2008.12.019
Sheih I-C, Fang TJ, Wu T-K, Lin P-H (2010) Anticancer and antioxidant activities of the peptide fraction from algae protein waste. J Agric Food Chem 58:1202–1207. https://doi.org/10.1021/jf903089m
Ko S-C, Kang N, Kim E-A et al (2012) A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem 47:2005–2011. https://doi.org/10.1016/j.procbio.2012.07.015
Zhang B, Zhang X (2013) Separation and nanoencapsulation of antitumor polypeptide from Spirulina platensis. Biotechnol Prog 29:1230–1238. https://doi.org/10.1002/btpr.1769
FAO (2020) Food and Agriculture Organization. Crop Information. http://www.fao.org/land-water/databases-and-software/crop-information/soybean/en/
Wollmann F, Dietze S, Ackermann J et al (2019) Microalgae wastewater treatment: biological and technological approaches. Eng Life Sci 19:860–871. https://doi.org/10.1002/elsc.201900071
Markou G, Wang L, Ye J, Unc A (2018) Using agro-industrial wastes for the cultivation of microalgae and duckweeds: contamination risks and biomass safety concerns. Biotechnol Adv 36:1238–1254. https://doi.org/10.1016/j.biotechadv.2018.04.003
Feng L, Casas ME, Ottosen LDM et al (2017) Removal of antibiotics during the anaerobic digestion of pig manure. Sci Total Environ 603–604:219–225. https://doi.org/10.1016/j.scitotenv.2017.05.280
Moerland MJ, Borneman A, Chatzopoulos P et al (2020) Increased (antibiotic-resistant) pathogen indicator organism removal during (hyper-) thermophilic anaerobic digestion of concentrated black water for safe nutrient recovery. Sustainability 12:9336. https://doi.org/10.3390/su12229336
Gurmessa B, Pedretti EF, Cocco S et al (2020) Manure anaerobic digestion effects and the role of pre- and post-treatments on veterinary antibiotics and antibiotic resistance genes removal efficiency. Sci Total Environ 721:137532. https://doi.org/10.1016/j.scitotenv.2020.137532
Mezzari MP, Prandini JM, Deon Kich J, Busi da Silva ML (2017) Elimination of antibiotic multi-resistant Salmonella typhimurium from swine wastewater by microalgae-induced antibacterial mechanisms. J Bioremediation Biodegrad 08: https://doi.org/10.4172/2155-6199.1000379
Mohsenpour SF, Hennige S, Willoughby N et al (2021) Integrating micro-algae into wastewater treatment: a review. Sci Total Environ 752:142168. https://doi.org/10.1016/j.scitotenv.2020.142168
Markou G, Wang L, Ye J, Unc A (2019) Cultivation of microalgae on anaerobically digested agro-industrial wastes and by-products. In: Application of microalgae in wastewater treatment. Springer International Publishing, Cham, pp 147–172
Acknowledgments
The authors gratefully acknowledge the support provided by CAPES Foundation, Ministry of Education of Brazil.
Funding
This study was supported by SISTRATES-BNDES (Project No 23.17.00.023.00.00).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Code availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Michelon, W., da Silva, M.L.B., Matthiensen, A. et al. Amino acids, fatty acids, and peptides in microalgae biomass harvested from phycoremediation of swine wastewaters. Biomass Conv. Bioref. 12, 869–880 (2022). https://doi.org/10.1007/s13399-020-01263-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01263-2