Abstract
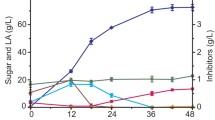
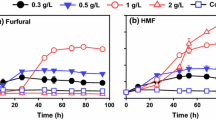
Expensive cellulase and complex detoxification procedures increase the cost of biomass lactic acid fermentation. Therefore, it is of great significance to develop a robust method to ferment lactic acid using biomass by avoiding cellulase and detoxification. This study demonstrates the advantage of combining mechanocatalytic P2O5 pre-treatment and strain domestication. We show that an enzyme-free mechanocatalytic saccharification process by combining mix-milling of P2O5 with biomass and successive hydrolysis produces a fermentable hydrolysate with much less inhibitory compounds than the hydrolysates obtained by conventional methods; only 5-HMF, furfural and acetic acid were detected in the biomass hydrolysate, and no phenolic inhibitors were detected. Pretreatment of biomass with P2O5 not only avoided cellulase, but also obtained less toxic hydrolysate. Furthermore, the Pediococcus pentosaceus strain gained superior inhibitor tolerance through domestication. It could tolerate 17.1 g/L acetic acid, 12.5 g/L 5-HMF, 11.9 g/L guaiacol and 11.5 g/L furfural and showed activity in decomposing furfural and 5-HMF for self-detoxification, allowing efficient lactic acid fermentation from biomass hydrolysate without detoxification. The lactic acid concentration and conversion rate fermented by domesticated bacteria were increased by 113.5% and 22.4%, respectively. In addition, the domesticated bacteria could utilize glucose and xylose simultaneously to produce lactic acid selectively. The combination of P2O5 pre-treatment and strain domestication to ferment lactic acid is applied to several biomass feedstocks, including corn stalk, corn stalk residue and rice husk residue. Lactic acid concentrations of 29.8 g/L, 31.1 g/L, and 46.2 g/L were produced from the hydrolysates of corn stalk, corn stalk residue and rice husk residue, respectively.






Similar content being viewed by others
References
Komesu A, Oliveira JA, Martins LH, Wolf Maciel MR, Maciel Filho R (2017) Lactic acid production to purification: a review. BioResources 12:4364–4383
Madhavan Nampoothiri K, Nair NR, John RP (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101:8493–8501
Detyothin S, Selke SE, Narayan R, Rubino M, Auras R (2013) Reactive functionalization of poly (lactic acid), PLA: Effects of the reactive modifier, initiator and processing conditions on the final grafted maleic anhydride content and molecular weight of PLA. Polym Degrad Stab 98:2697–2708
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2011) Lactic acid from lignocellulose-derived sugars using lactic acid bacteria: overview and limits. J Biotechnol 156:286–301
Wang LM, Zhao B, Liu B, Yang CY, Yu B, Li QG, Ma CQ, Xu P, Ma YH (2010) Efficient production of L-lactic acid from cassava powder by Lactobacillus rhamnosus. Bioresour Technol 101:7895–7901
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902
Bilal M, Asgher M, Iqbal HM, Hu H, Zhang X (2017) Biotransformation of lignocellulosic materials into value-added products-a review. Int J Biol Macromol 98:447–458
Arevalo-Gallegos A, Ahmad Z, Asgher M, Parra-Saldivar R, Iqbal HM (2017) Lignocellulose: a sustainable material to produce value-added products with a zero waste approach-a review. Int J Biol Macromol 99:308–318
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642
Ragauskas AJ, Williams CK, Davison BH, Britovsek G (2006) The path forward for biofuels and biomaterials. Science 311:484–489
Zhang Z, Xie Y, He X, Li X, Hu J, Ruan Z, Zhao S, Peng N, Liang Y (2016) Comparison of high-titer lactic acid fermentation from NaOH and NH3-H2O2-pretreated corncob by Bacillus coagulans using simultaneous saccharifcation and fermentation. Sci Rep 6:37245
Aulitto M, Fusco S, Nickel DB, Bartolucci S, Contursi P, Franzén CJ (2019) Seed culture pre-adaptation of Bacillus coagulans MA-13 improves LA production in simultaneous saccharifcation and fermentation. Biotechnol Biofuels 12:45
Kumar BDM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729
Chundawat SP, Beckham GT, Himmel ME, Dale BE (2011) Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu Rev Chem Biomol 2:121–145
Alvira P, Tomás-Pejó E, Ballesteros M, Negro M (2010) Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol 101:4851–4861
Van DJ, Pletschke B (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes-factors afecting enzymes, conversion and synergy. Biotechnol Adv 30:1458–1480
Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA (2012) The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng 109:1083–1087
Parawira W, Tekere M (2011) Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production. Crit Rev Biotechnol 31:20–31
Palmqvist E, Hahn-Hagerdal B (2000) Fermentation of lignocellulosic hydrolysates II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Almeida JR, Modig T, Petersson A, Hähn-Hägerdal B, Lidén G, Gorwa-Grauslund MF (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol 82:340–349
Jönsson LJ, Martín C (2016) Pretreatment of lignocellulose: formation of inhibitory byproducts and strategies for minimizing their effects. Bioresour Technol 199:103–112
Hu JL, Lin YX, Zhang ZT, Xiang T, Mei YX, Zhao SM, Liang YX, Peng N (2015) High-titer lactic acid production from NaOH-pretreated corn stover by Bacillus coagulans LA204 using fed-batch simultaneous saccharification and fermentation under non-sterile condition. Bioresour Technol 182:251–257
Zhao K, Qiao Q, Chu D, Gu H, Dao TD, Zhang J, Bao J (2013) Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresour Technol 135:481–489
Xie YJ, Hu Q, Feng GD, Jiang X, Hu JL, He MX, Hu GQ, Zhao SM, Liang YX, Ruan ZY, Peng N (2018) Biodetoxifification of phenolic inhibitors from lignocellulose pretreatment using Kurthia huakuii LAM0618T and subsequent lactic acid fermentation. Molecules 23:2626
Choi SY, Gong G, Park HS, Um Y, Sim SJ, Woo HM (2015) Extreme furfural tolerance of a soil bacterium Enterobacter cloacae GGT036. J Biotechnol 193:11–13
Fonseca BG, Moutta Rde O, Ferraz Fde O, Vieira ER, Nogueira AS, Baratella BF, Rodrigues LC, Hou-Rui Z, da Silva SS (2011) Biological detoxifification of different hemicellulosic hydrolysates using Issatchenkia occidentalis CCTCC M 206097 yeast. J Ind Microbiol Biotechnol 38:199–207
Huang C, Wu H, Smith TJ, Liu ZJ, Lou WY, Zong MH (2012) In vivo detoxification of furfural during lipid production by the oleaginous yeast Trichosporon fermentans. Biotechnol Lett 34:1637–1642
Ran H, Zhang J, Gao Q, Lin Z, Bao J (2014) Analysis of biodegradation performance of furfural and 5-hydroxymethylfurfural by Amorphotheca resinae ZN1. Biotechnol Biofuels 7:51
Sun Y, Yang Y, Liu H, Wei C, Qi W, Xiu Z (2020) Simultaneous liquefaction, saccharification, and fermentation of l-lactic acid using aging paddy rice with hull by an isolated thermotolerant Enterococcus faecalis DUT1805. Bioproc Biosyst Eng 43:1717–1724
Oliveira RA, Rossell CE, Venus J, Rabelo SC, Filho RM (2018) Detoxification of sugarcane-derived hemicellulosic hydrolysate using a lactic acid producing strain. J Biotechnol 278:56–63
Mussatto SI, Roberto IC (2004) Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresour Technol 93:1–10
Guo W, Jia W, Li Y, Chen S (2010) Performances of Lactobacillus brevis for producing lactic acid from hydrolysate of lignocellulosics. Appl Biochem Biotech 161:124–136
Mills TY, Sandoval NR, Gill RT (2009) Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol Biofuels 2:26
Van der Pol E, Springer J, Vriesendorp B, Weusthuis R, Eggink G (2016) Precultivation of Bacillus coagulans DSM2314 in the presence of furfural decreases inhibitory effects of lignocellulosic by-products during l (+)-lactic acid fermentation. Appl Microbiol Biotechnol 100:10307–10319
Van der Pol EC, Vaessen E, Weusthuis RA, Eggink G (2016) Identifying inhibitory effects of lignocellulosic by-products on growth of lactic acid producing micro-organisms using a rapid small-scale screening method. Biores Technol 209:297–304
Van der Pol EC, Bakker RR, Baets P, Eggink G (2014) By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Appl Microbiol Biotechnol 98:9579–9593
Van der Pol EC, Bakker RR, van Zeeland ANT, Sanchez Garcia D, Punt A, Eggink G (2015) Analysis of by-product formation and sugar monomerization in sugarcane bagasse pretreated at pilot plant scale: differences between autohydrolysis, alkaline and acid pretreatment. Bioresour Technol 181:114–123
Liu X, Liu H, Yan P, Mao L, Xu Z, Zhang Z (2020) Mechanocatalytic synergy for expedited cellulosic ethanol production compatible with integrated biorefinery. ACS Sustain Chem Eng 8:2399–2408
Zhang Z, Xie Y, He X, Li X, Hu J, Ruan Z, Zhao S, Peng N, Liang Y (2016) Comparison of high-titer lactic acid fermentation from NaOH- and NH3 -H2O2 -pretreated corncob by Bacillus coagulans using simultaneous saccharifification and fermentation. Sci Rep 6:37245
Jonsson LJ, Alriksson B, Nilvebrant NO (2013) Bioconversion of lignocellulose: Inhibitors and detoxifification. Biotechnol Biofuels 6:16
Toquero C, Bolado S (2014) Effect of four pretreatments on enzymatic hydrolysis and ethanol fermentation of wheat straw. Influence of inhibitors and washing. Bioresour Technol 157:68–76
Chen X, Xue Y, Hu J, Tsang YF, Gao MT (2017) Release of polyphenols is the major factor influencing the bioconversion of rice straw to lactic acid. Appl Biochem Biotech 183:685–698
Abdel-Rahman MA, Sonomoto K (2016) Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J Biotechnol 236:176–192
Tomás-Pejó E, Olsson L (2015) Infuence of the propagation strategy for obtaining robust Saccharomyces cerevisiae cells that effciently co-ferment xylose and glucose in lignocellulosic hydrolysates. Microb Biotechnol 8:999–1005
Nielsen F, Tomás-Pejó E, Olsson L, Wallberg O (2015) Short-term adaptation during propagation improves the performance of xylose-fermenting Saccharomyces cerevisiae in simultaneous saccharifcation and co-fermentation. Biotechnol Biofuels 8:219
Wikandari R, Millati R, Syamsiyah S, Muriana R, Ayuningsih Y (2010) Effect of furfural, hydroxymethylfurfural and acetic acid on indigeneous microbial isolate for bioethanol production. Agric J 5:105–109
Zhang Y, Han B, Ezeji TC (2012) Biotransformation of furfural and 5-hydroxymethyl furfural (HMF) by Clostridium acetobutylicum ATCC 824 during butanol fermentation. N Biotechnol 29:345–351
Palmqvist E, Hahn-Hagerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74:17–24
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26
Acknowledgements
This research was supported by National Natural Science Foundation of China (21932005, 21721004, 21690084).
Author information
Authors and Affiliations
Contributions
[ZCZ] conceived the overall project. [HL] carried out all experiments, data analysis was performed by [HL] and [XL], the first draft of the manuscript was written by [HL] and all authors contributed to the discussion and comments on the preparation of manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Liu, X., Jiang, H. et al. Enhanced lactic acid production from P2O5-pretreated biomass by domesticated Pediococcus pentosaceus without detoxification. Bioprocess Biosyst Eng 44, 2153–2166 (2021). https://doi.org/10.1007/s00449-021-02591-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02591-x