Abstract
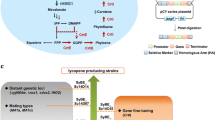
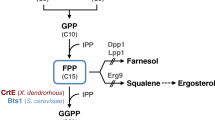
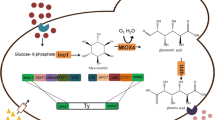
To construct a Saccharomyces cerevisiae strain for efficient lycopene production, we used a pathway engineering strategy based on expression modules comprising fusion proteins and a strong constitutive promoter. The two recombinant plasmids pEBI encoding the fusion genes with an inducible promoter, as well as pIETB with a constitutive promoter and terminator were introduced into S. cerevisiae YPH499 and BY4741 to obtain the four recombinant strains ypEBI, ypIETB, byEBI and byIETB. The lycopene production and the transcription levels of key genes were higher in the BY4741 chassis than in YPH499. Accordingly, the content of total and unsaturated fatty acids was also higher in BY4741, which also exhibited a decrease of glucose, increase of trehalose, increase of metabolite in citrate cycle, and low levels of amino acids. These changes rerouted metabolic fluxes toward lycopene synthesis, indicating that the BY4741 chassis was more suitable for lycopene synthesis. The lycopene content of bpIETB in SG-Leu medium supplemented with 100 mg/L of linolenic acid reached 10.12 mg/g dry cell weight (DCW), which was 85.7% higher than without the addition of unsaturated fatty acids. The constitutive promoter expression strategy employed in this study achieved efficient lycopene synthesis in S. cerevisiae, and the strain bpIETB was obtained a suitable chassis host for lycopene production, which provides a basis for further optimization of lycopene production in artificial synthetic cells and a reference for the multi-enzyme synthesis of other similar complex terpenoids.









Similar content being viewed by others
References
Moise AR, Al-Babili S, Wurtzel ET (2013) Mechanistic aspects of carotenoid biosynthesis. Chem Rev 114(1):164–193
Müller L, Caris-Veyrat C, Lowe G, Böhm V (2016) Lycopene and its antioxidant role in the prevention of cardiovascular diseases—a critical review. Crit Rev Food Sci Nutr 56(11):1868–1879
Xu X, Tian L, Xu J, Xie C, Jiang L, Huang H (2018) Analysis and expression of the carotenoid biosynthesis genes from Deinococcus wulumuqiensis R12 in engineered Escherichia coli. AMB Express 8(1):94
Ma T, Deng Z, Liu T (2016) Microbial production strategies and applications of lycopene and other terpenoids. World J Microb Biot 32(1):15
Hernández-Almanza A, Montañez J, Martínez G, Aguilar-Jiménez A, Contreras-Esquivel JC, Aguilar CN (2016) Lycopene: progress in microbial production. Trends Food Sci Technol 56:142–148
Chen H, Liu C, Li M, Zhang H, Xian M, Liu H (2018) Directed evolution of mevalonate kinase in Escherichia coli by random mutagenesis for improved lycopene. RSC Adv 8(27):15021–15028
Li X, Wang Z, Zhang G, Yi L (2019) Improving lycopene production in Saccharomyces cerevisiae through optimizing pathway and chassis metabolism. Chem Eng Sci 193:364–369
Schwartz C, Frogue K, Misa J, Wheeldon I (2017) Host and pathway engineering for enhanced lycopene biosynthesis in Yarrowia lipolytica. Front Microbiol 8:2233
Wang Y, Pang J, Zheng Y, Jiang P, Gong W, Chen X, Chen D (2017) Genetic manipulation of the bifunctional gene, carRA, to enhance lycopene content in Blakeslea trispora. Biochem Eng J 119:27–33
Ignea C, Cvetkovic I, Loupassaki S, Kefalas P, Johnson CB, Kampranis SC, Makris AM (2011) Improving yeast strains using recyclable integration cassettes, for the production of plant terpenoids. Microb Cell Fact 10(1):4
Bahieldin A, Gadalla NO, Al-Garni SM, Almehdar H, Noor S, Hassan SM, Shokry AM, Sabir JSM, Murata N (2014) Efficient production of lycopene in Saccharomyces cerevisiae by expression of synthetic crt genes from a plasmid harboring the ADH2 promoter. Plasmid 72:18–28
Chen Y, Xiao W, Wang Y, Liu H, Li X, Yuan Y (2016) Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb Cell Fact 15(1):113
Xie W, Lv X, Ye L, Zhou P, Yu H (2015) Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab Eng 30:69–78
Shi B, Ma T, Ye Z, Li X, Huang Y, Zhou Z, Ding Y, Deng Z, Liu T (2019) Systematic metabolic engineering of Saccharomyces cerevisiae for lycopene overproduction. J Agric Food Chem 67(40):11148–11157
Sun Y, Sun L, Shang F, Yan G (2016) Enhanced production of beta-carotene in recombinant Saccharomyces cerevisiae by inverse metabolic engineering with supplementation of unsaturated fatty acids. Process Biochem 51(5):568–577
Vereschagina OA, Memorskaya AS, Tereshina VM (2010) The role of exogenous lipids in lycopene synthesis in the mucoraceous fungus Blakeslea trispora. Microbiology 79(5):593–601
Luo Z, Liu N, Lazar Z, Chatzivasileiou A, Ward V, Chen J, Zhou J, Stephanopoulos G (2020) Enhancing isoprenoid synthesis in Yarrowia lipolytica by expressing the isopentenol utilization pathway and modulating intracellular hydrophobicity. Metab Eng 61:344–351
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Cahoon EB, Ripp KG, Hall SE, Kinney AJ (2001) Formation of conjugated Δ8, Δ10-double bonds by Δ12-oleic-acid desaturase-related enzymes. J Biol Chem 276(4):2637–2643
Ren LJ, Chen SL, Geng LJ, Ji XJ, Xu X, Song P, Gao S, Huang H (2018) Exploring the function of acyltransferase and domain replacement in order to change the polyunsaturated fatty acid profile of Schizochytrium sp. Algal Res 29:193–201
Wu Q, Zhu L, Xu Q, Huang H, Jiang L, Yang ST (2017) Tailoring the oxidative stress tolerance of Clostridium tyrobutyricum CCTCC W428 by introducing trehalose biosynthetic capability. J Agric Food Chem 65(40):8892–8901
Chong J, Wishart DS, Xia J (2019) Using metaboanalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr Protoc Bioinform 68(1):e86
Liang J, Ning JC, Zhao H (2013) Coordinated induction of multi-gene pathways in Saccharomyces cerevisiae. Nucleic Acids Res 41(4):e54–e54
Wang Y, Yi H, Wang M, Yu O, Jez JM (2011) Structural and kinetic analysis of the unnatural fusion protein 4-coumaroyl-CoA Ligase::stilbene synthase. J Am Chem Soc 133(51):20684–20687
Shen HJ, Hu JJ, Li XR, Liu JZ (2015) Engineering of Escherichia coli for lycopene production through promoter engineering. Curr Pharm Biotechnol 16(12):1094–1103
Wang Z, Wei L, Sheng Y, Zhang G (2019) Yeast synthetic terminators: fine regulation of strength through linker sequences. ChemBioChem 20(18):2383–2389
Xie W, Ye L, Lv X, Xu H, Yu H (2015) Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae. Metab Eng 28:8–18
Wei Y, Mohsin A, Hong Q, Guo M, Fang H (2018) Enhanced production of biosynthesized lycopene via heterogenous MVA pathway based on chromosomal multiple position integration strategy plus plasmid systems in Escherichia coli. Bioresour Technol 250:382–389
Ma T, Shi B, Ye Z, Li X, Liu M, Chen Y, Xia J, Nielsen J, Deng Z, Liu T (2019) Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab Eng 52:134–142
Xu X, Jin W, Jiang L, Xu Q, Li S, Zhang Z, Huang H (2016) A high-throughput screening method for identifying lycopene-overproducing E. coli strain based on an antioxidant capacity assay. Biochem Eng J 112:277–284
Westfall PJ, Pitera DJ, Lenihan JR, Eng D, Woolard FX, Regentin R, Horning T, Tsuruta H, Melis DJ, Owens A, Fickes S, Diola D, Benjamin KR, Keasling JD, Leavell MD, McPhee DJ, Renninger NS, Newman JD, Paddon CJ (2012) Production of amorphadiene in yeast, and its conversion to dihydroartemisinic acid, precursor to the antimalarial agent artemisinin. Proc Natl Acad Sci USA 109:E111–E118
Xu J, Xu X, Xu Q, Zhang Z, Jiang L, Huang H (2018) Efficient production of lycopene by engineered E. coli strains harboring different types of plasmids. Bioproc Biosyst Eng 41(4):489–499
Larroude M, Celinska E, Back A, Thomas S, Nicaud J-M, Ledesma-Amaro R (2018) A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol Bioeng 115(2):464–472
Maury J, Asadollahi MA, Møller K, Schalk M, Clark A, Formenti LR, Nielsen J (2008) Reconstruction of a bacterial isoprenoid biosynthetic pathway in Saccharomyces cerevisiae. FEBS Lett 582(29):4032–4038
Lv X, Xie W, Lu W, Guo F, Gu J, Yu H, Ye L (2014) Enhanced isoprene biosynthesis in Saccharomyces cerevisiae by engineering of the native acetyl-CoA and mevalonic acid pathways with a push–pull–restrain strategy. J Biotechnol 186:128–136
Luo W, Gong Z, Li N, Zhao Y, Zhang H, Yang X, Liu Y, Rao Z, Yu X, Druzhinina IS (2020) A negative regulator of carotenogenesis in Blakeslea trispora. Appl Environ Microb 86(6):e02462-e2519
Jin M, Xiao A, Zhu L, Zhang Z, Huang H, Jiang L (2019) The diversity and commonalities of the radiation-resistance mechanisms of Deinococcus and its up-to-date applications. AMB Express 9(1):138
He Z, Wang S, Yang Y, Hu J, Wang C, Li H, Ma B, Yuan Q (2017) β-Carotene production promoted by ethylene in Blakeslea trispora and the mechanism involved in metabolic responses. Process Biochem 57:57–63
Zhao C, Gao Q, Chen J, Wei L, Imanaka T, Hua Q (2017) Metabolomic changes and metabolic responses to expression of heterologous biosynthetic genes for lycopene production in Yarrowia lipolytica. J Biotechnol 251:174–185
Alcalde E, Fraser PD (2018) Extending our tools and resources in the non-conventional industrial yeast Xanthophyllomyces dendrorhous through the application of metabolite profiling methodologies. Metabolomics 14(3):30
Bu X, Sun L, Shang F, Yan G (2017) Comparative metabolomics profiling of engineered Saccharomyces cerevisiae lead to a strategy that improving β-carotene production by acetate supplementation. PLoS ONE 12(11):e0188385
Verwaal R, Jiang Y, Wang J, Daran J-M, Sandmann G, van den Berg JA, van Ooyen AJJ (2010) Heterologous carotenoid production in Saccharomyces cerevisiae induces the pleiotropic drug resistance stress response. Yeast 27(12):983–998
Hu J, Li H, Yang Y, Wang S, Tang P, Li C, Tian G, Yuan Q (2015) Metabolic regulation of α-linolenic acid on β-carotene synthesis in Blakeslea trispora revealed by a GC-MS-based metabolomic approach. RSC Adv 5(78):63193–63201
Zhou P, Xie W, Li A, Wang F, Yao Z, Bian Q, Zhu Y, Yu H, Ye L (2017) Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzyme Microb Technol 100:28–36
Verwaal R, Wang J, Meijnen J-P, Visser H, Sandmann G, van den Berg JA, van Ooyen AJ (2007) High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Environ Microb 73(13):4342–4350
Shi F, Zhan W, Li Y, Wang X (2014) Temperature influences β-carotene production in recombinant Saccharomyces cerevisiae expressing carotenogenic genes from Phaffia rhodozyma. World J Microb Biot 30(1):125–133
Li Q, Sun Z, Li J, Zhang Y (2013) Enhancing beta-carotene production in Saccharomyces cerevisiae by metabolic engineering. FEMS Microbiol Lett 345(2):94–101
Gao S, Tong Y, Zhu L, Ge M, Jiang Y, Chen D, Yang S (2017) Production of β-carotene by expressing a heterologous multifunctional carotene synthase in Yarrowia lipolytica. Biotechnol Lett 39(6):921–927
Matthäus F, Ketelhot M, Gatter M, Barth G (2014) Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica. Appl Environ Microb 80(5):1660–1669
Bhataya A, Schmidt-Dannert C, Lee PC (2009) Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem 44(10):1095–1102
Araya-Garay JM, Feijoo-Siota L, Rosa-dos-Santos F, Veiga-Crespo P, Villa T (2012) Construction of new Pichia pastoris X-33 strains for production of lycopene and β-carotene. Appl Microbiol Biot 93(6):2483–2492
Zhang X, Wang D, Duan Y, Zheng X, Lin Y, Liang S (2020) Production of lycopene by metabolically engineered Pichia pastoris. Biosci Biotechnol Biochem 84(3):463–470
Li L, Liu Z, Jiang H, Mao X (2020) Biotechnological production of lycopene by microorganisms. Appl Microbiol Biot 104:10307–10324
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21978136), the National Natural Science Foundation of China Youth Fund (31922070), the Natural Science Foundation of Jiangsu Province (BK20180038, BK20171461), and Basic Scientific R&D Program for Public Welfare Institutes in Xinjiang (KY2019023).
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, X., Liu, J., Lu, Y. et al. Pathway engineering of Saccharomyces cerevisiae for efficient lycopene production. Bioprocess Biosyst Eng 44, 1033–1047 (2021). https://doi.org/10.1007/s00449-020-02503-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02503-5