Abstract
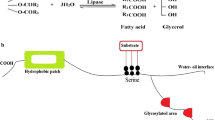
Lipases CAL-B, TLL, and RML were used in the synthesis of free fatty acids of grape seed oil as heterogeneous substrate. The best enzyme was used to optimize the reaction variables temperature, enzyme content, and molar ratio of water:oil in batch reactions using experimental planning. The ideal conditions to produce free fatty acids using pure RML were 45 °C, 12:1 substrate molar ratio, and 15% enzyme, resulting in 66% of oil hydrolysis and a productivity of 0.54 mol L−1 min−1 in 4 h of reaction at 180 rpm. Repeated batches of reaction were performed testing the operational stability of RML, results showing that this enzyme could be used for at least 20 cycles keeping more than 80% of its initial activity, suggesting its potential use in industrial processes. The synthesis of free fatty acids was then evaluated in continuous reactions using packed-bed reactor (PBR). The highest productivity in the continuous process was 6.85 mol L−1 min−1, using only RML, showing an operational stability higher than 80% of its initial conversion capacity after 11 days of operation, at a flow rate of 0.13 mL min−1 at 45 °C. We evaluated the use of this hydrolyzed oil as substrate for lactone bioproduction using Galactomyces geotrichum UFMG-CM-Y3276, G. geotrichum UFMG-CM-Y3558, and Geotrichum klebahnii UFMG-CM-Y3014 screened for their oil-hydrolysis ability. Volatile compounds were qualitatively identified in GC–MS as γ-octalactone and γ-nonalactone.





Similar content being viewed by others
References
Van Leeuwen C, Friant P, Choné X et al (2004) Influence of climate, soil, and cultivar on terroir. Am J Enol Vitic 55:207–217
Fiori L (2007) Grape seed oil supercritical extraction kinetic and solubility data: critical approach and modeling. J Supercrit Fluids 43:43–54. https://doi.org/10.1016/j.supflu.2007.04.009
Maier T, Schieber A, Kammerer DR, Carle R (2009) Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem 112:551–559. https://doi.org/10.1016/j.foodchem.2008.06.005
Bhosle BM, Subramanian R (2005) New approaches in deacidification of edible oils—a review. J Food Eng 69:481–494. https://doi.org/10.1016/j.jfoodeng.2004.09.003
de Haro JC, Izarra I, Rodríguez JF et al (2016) Modelling the epoxidation reaction of grape seed oil by peracetic acid. J Clean Prod 138:70–76. https://doi.org/10.1016/j.jclepro.2016.05.015
Passos CP, Silva RM, Da Silva FA et al (2010) Supercritical fluid extraction of grape seed (Vitis vinifera L.) oil: effect of the operating conditions upon oil composition and antioxidant capacity. Chem Eng J 160:634–640. https://doi.org/10.1016/j.cej.2010.03.087
Crews C, Hough P, Brereton P et al (2006) Quantitation of the main constituents of some authentic sesame seed oils of different origin. J Agric Food Chem 54:6266–6270. https://doi.org/10.1021/jf0603578
Villeneuve P, Muderhwa JM, Graille J, Haas MJ (2000) Customizing lipases for biocatalysis: a survey of chemical, physical and molecular biological approaches. J Mol Catal B Enzym 9:113–148. https://doi.org/10.1016/S1381-1177(99)00107-1
Mendes AA, de Oliveira PC, de Castro HF, Giordano RDLC (2011) Application of chitosan as support for immobilization of enzymes of industrial interest. Quim Nova 34:831–840. https://doi.org/10.1590/S0100-40422011000500019
Ribeiro BD, de Castro AM, Coelho MAZ, Freire DMG (2011) Production and use of lipases in bioenergy: a review from the feedstocks to biodiesel production. Enzyme Res 2011:1–16. https://doi.org/10.4061/2011/615803
Coelho AD, Santos KC, Domingues RCC, Mendes AA (2013) Produção de concentrados de ácidos graxos por hidrólise de óleos vegetais mediada por lipase vegetal. Quim Nova 36:1164–1169. https://doi.org/10.1590/S0100-40422013000800015
Dalla-Vecchia R, Nascimento MDG, Soldi V (2004) Aplicações sintéticas de lipases imobilizadas em polímeros. Quim Nova 27:623–630. https://doi.org/10.1590/s0100-40422004000400017
Palomo JM, Muoz G, Fernández-Lorente G et al (2002) Interfacial adsorption of lipases on very hydrophobic support (octadecyl-Sepabeads): immobilization, hyperactivation and stabilization of the open form of lipases. J Mol Catal B Enzym 19:279–286. https://doi.org/10.1016/S1381-1177(02)00178-9
Rodrigues RC, Ayub MAZ (2011) Effects of the combined use of Thermomyces lanuginosus and Rhizomucor miehei lipases for the transesterification and hydrolysis of soybean oil. Process Biochem 46:682–688. https://doi.org/10.1016/j.procbio.2010.11.013
Alves JS, Vieira NS, Cunha AS et al (2014) Combi-lipase for heterogeneous substrates: a new approach for hydrolysis of soybean oil using mixtures of biocatalysts. RSC Adv 4:6863–6868. https://doi.org/10.1039/c3ra45969a
Séverac E, Galy O, Turon F et al (2011) Selection of CalB immobilization method to be used in continuous oil transesterification: analysis of the economical impact. Enzyme Microb Technol 48:61–70. https://doi.org/10.1016/j.enzmictec.2010.09.008
Poppe JK, Fernandez-Lafuente R, Rodrigues RC, Ayub MAZ (2015) Enzymatic reactors for biodiesel synthesis: present status and future prospects. Biotechnol Adv 33:511–525. https://doi.org/10.1016/j.biotechadv.2015.01.011
Hama S, Tamalampudi S, Yoshida A et al (2011) Enzymatic packed-bed reactor integrated with glycerol-separating system for solvent-free production of biodiesel fuel. Biochem Eng J 55:66–71. https://doi.org/10.1016/j.bej.2011.03.008
Matte CR, Bordinhão C, Poppe JK et al (2016) Synthesis of butyl butyrate in batch and continuous enzymatic reactors using Thermomyces lanuginosus lipase immobilized in Immobead 150. J Mol Catal B Enzym 127:67–75. https://doi.org/10.1016/j.molcatb.2016.02.016
Poppe JK, Matte CR, de Freitas VO et al (2018) Enzymatic synthesis of ethyl esters from waste–oil using mixtures of lipases in a plug-flow packed-bed continuous reactor. Biotechnol Prog 34:952–959. https://doi.org/10.1002/btpr.2650
Neto RS, Pastore GM, Macedo GA (2006) Biocatalysis and biotransformation producing γ-decalactone. J Food Sci 69:C677–C680. https://doi.org/10.1111/j.1365-2621.2004.tb09914.x
Romero-Guido C, Belo I, Ta TMN et al (2011) Biochemistry of lactone formation in yeast and fungi and its utilisation for the production of flavour and fragrance compounds. Appl Microbiol Biotechnol 89:535–547. https://doi.org/10.1007/s00253-010-2945-0
Endrizzi A, Pagot Y, Le Clainche A et al (1996) Production of lactones and peroxisomal beta-oxidation in yeasts. Crit Rev Biotechnol 16:301–329. https://doi.org/10.3109/07388559609147424
Rabenhorst J, Gatfield I (2002) Method of producing γ-decalactone using Yarrowia lipolytica strain HR 145 (DSM 12397). US6451565B1, United States Patent. https://patents.google.com/patent/US6451565B1/en
Grondin E, Sing ASC, James S et al (2017) Flavour production by Saprochaete and Geotrichum yeasts and their close relatives. Food Chem 237:677–684. https://doi.org/10.1016/j.foodchem.2017.06.009
de Freitas VO, Matte CR, Poppe JK et al (2019) Ultrasound-assisted transesterification of soybean oil using combi-lipase biocatalysts. Braz J Chem Eng 36:995–1005. https://doi.org/10.1590/0104-6632.20190362s20180455
Poppe JK, Matte CR, Fernandez-Lafuente R et al (2018) Transesterification of waste frying oil and soybean oil by combi-lipases under ultrasound-assisted reactions. Appl Biochem Biotechnol 186:576–589. https://doi.org/10.1007/s12010-018-2763-x
Poppe JK, Matte CR, do Peralba CRM et al (2015) Optimization of ethyl ester production from olive and palm oils using mixtures of immobilized lipases. Appl Catal A Gen 490:50–56. https://doi.org/10.1016/j.apcata.2014.10.050
Poppe JK, Garcia-Galan C, Matte CR et al (2013) Optimization of synthesis of fatty acid methyl esters catalyzed by lipase B from Candida antarctica immobilized on hydrophobic supports. J Mol Catal B Enzym 94:51–56. https://doi.org/10.1016/j.molcatb.2013.05.010
van Den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr A 11:463–471. https://doi.org/10.1016/s0021-9673(01)80947-x
Vaysse L, Ly A, Moulin G, Dubreucq E (2002) Chain-length selectivity of various lipases during hydrolysis, esterification and alcoholysis in biphasic aqueous medium. Enzyme Microb Technol 31:648–655. https://doi.org/10.1016/S0141-0229(02)00166-7
Pleiss J, Fischer M, Schmid RD (1998) Anatomy of lipase binding sites: the scissile fatty acid binding site. Chem Phys Lipids 93:67–80. https://doi.org/10.1016/S0009-3084(98)00030-9
Avelar MHM, Cassimiro DMJ, Santos KC et al (2013) Hydrolysis of vegetable oils catalyzed by lipase extract powder from dormant castor bean seeds. Ind Crops Prod 44:452–458. https://doi.org/10.1016/j.indcrop.2012.10.011
Xu Y, Guo S, Wang W et al (2013) Enzymatic hydrolysis of palm stearin to produce diacylglycerol with a highly thermostable lipase. Eur J Lipid Sci Technol 115:564–570. https://doi.org/10.1002/ejlt.201200373
Cossignani L, Damiani P, Simonetti MS, Maňes J (2004) Biocatalyzed acidolysis of soybean oil triacylglycerols to increase oleic acid content. J Chromatogr A 1052:167–170. https://doi.org/10.1016/j.chroma.2004.08.015
Martins AB, Graebin NG, Lorenzoni ASG et al (2011) Rapid and high yields of synthesis of butyl acetate catalyzed by Novozym 435: reaction optimization by response surface methodology. Process Biochem 46:2311–2316. https://doi.org/10.1016/j.procbio.2011.09.011
Rodrigues RC, Volpato G, Wada K, Ayub MAZ (2008) Enzymatic synthesis of biodiesel from transesterification reactions of vegetable oils and short chain alcohols. JAOCS J Am Oil Chem Soc 85:925–930. https://doi.org/10.1007/s11746-008-1284-0
Nie K, Xie F, Wang F, Tan T (2006) Lipase catalyzed methanolysis to produce biodiesel: optimization of the biodiesel production. J Mol Catal B Enzym 43:142–147. https://doi.org/10.1016/j.molcatb.2006.07.016
Halim SFA, Kamaruddin AH, Fernando WJN (2009) Continuous biosynthesis of biodiesel from waste cooking palm oil in a packed bed reactor: optimization using response surface methodology (RSM) and mass transfer studies. Bioresour Technol 100:710–716. https://doi.org/10.1016/j.biortech.2008.07.031
Damstrup ML, Kiil S, Jensen AD et al (2007) Process development of continuous glycerolysis in an immobilized enzyme-packed reactor for industrial monoacylglycerol production. J Agric Food Chem 55:7786–7792. https://doi.org/10.1021/jf063366p
Rico ALL, de Castro HF, de Oliveira PC (2015) Produção enzimática de biodiesel em reator de leito fixo e fluxo contínuo utilizando células íntegras de Mucor circinelloides imobilizadas em espuma de poliuretano. In: Anais do XX Congresso Brasileiro de Engenharia Química, vol. 1. Blucher Chemical Engineering Proceedings, São Paulo, pp 2437–2441. https://doi.org/10.5151/chemeng-cobeq2014-1730-17852-15131
Kwiatkowska B, Bennett J, Akunna J et al (2011) Stimulation of bioprocesses by ultrasound. Biotechnol Adv 29:768–780. https://doi.org/10.1016/j.biotechadv.2011.06.005
Fallavena LP, Antunes FHF, Alves JS et al (2014) Ultrasound technology and molecular sieves improve the thermodynamically controlled esterification of butyric acid mediated by immobilized lipase from Rhizomucor miehei. RSC Adv 4:8675–8681. https://doi.org/10.1039/c3ra47315e
Świzdor A, Panek A, Milecka-Tronina N, Kołek T (2012) Biotransformations utilizing β-oxidation cycle reactions in the synthesis of natural compounds and medicines. Int J Mol Sci 13:16514–16543. https://doi.org/10.3390/ijms131216514
Kourist R, Hilterhaus L (2015) Microbial lactone synthesis based on renewable resources. Mol Microbiol 20:275–301. https://doi.org/10.1007/978-3-662-45209-7_10
Farbood MI, Mclean LB, Morris JA, Bondarovich HA (1992) Octalactone-containing composition, fermentation process for producing same and organoleptic uses thereof. EP0519481A2, European Patent Office. https://patents.google.com/patent/EP0519481A2/en
Tress R, Apetz M, Arrieta R, Grünewald KG (1978) Formation of lactones and terpenoids by microorganisms. In: Charalambous G, Inglett G (eds) Flavor of Foods and Beverages. Elsevier, London, pp 145–168
Lange H, Garbe L (2000) Yeast alpha-oxidation enzymes, used to degrade organic compounds by one carbon atom, used to convert 5-hydroxydecanoic acid to gamma-nonalactone. DE19929577A1, German Patent and Trade Mark Office. https://patents.google.com/patent/DE19929577A1/en
Damasceno S, Cereda MP, Pastore GM, Oliveira JG (2003) Production of volatile compounds by Geotrichum fragrans using cassava wastewater as substrate. Process Biochem 39:411–414. https://doi.org/10.1016/S0032-9592(03)00097-9
Mdaini N, Gargouri M, Hammami M et al (2006) Production of natural fruity aroma by Geotrichum candidum. Appl Biochem Biotechnol 128:227–235. https://doi.org/10.1385/ABAB:128:3:227
Acknowledgements
This work was supported by grants from Brazilian Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, scholarships for the first and fifth author), Finance Code 001, and grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Grant # 442516/2014-2 for the corresponding author), and from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS). The authors would like to thank Mr. Ramiro Martinez (Novozymes, Spain S.A.) for kindly supplying the enzymes used in this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Castiglioni, G.Z., Bettio, G., Matte, C.R. et al. Production of volatile compounds by yeasts using hydrolysed grape seed oil obtained by immobilized lipases in continuous packed-bed reactors. Bioprocess Biosyst Eng 43, 1391–1402 (2020). https://doi.org/10.1007/s00449-020-02334-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02334-4