Abstract
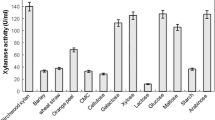
A high titre of thermo-alkali-stable xylanase was attained in cane molasses medium. When the culture variables for endoxylanase production were optimized [cane molasses 7 %, soluble alkaline extract of wheat bran (SAE-WB) 37 % and ammonium chloride 0.30 %], a 4.5-fold enhancement in xylanase production (69 U ml−1) was achieved as compared to that in the unoptimized medium (15 U ml−1). The enzyme titre attained in shake flasks could be sustained in a 7-l laboratory bioreactor. An activity band corresponding to 40 kDa was visualized on SDS-PAGE zymogram analysis. The enzyme has broad range of pH and temperature for activity with optima at 9.0 and 80 °C, and stable between pH 4.0 and 11.0 with 85 % retention of activity. It has T 1/2 of 40 and 15 min at 70 and 80 °C. The enzyme is halotolerant since it displays activity in the presence of salt up to 15 %, and remains 100 % active in the absence of salt. The supplementation of whole wheat dough with xylanase improves antistaling property, reducing sugar content, bread volume with prebiotic xylooligosaccharides in bread. This is the first report on xylanase production in cane molasses medium with SAE-WB as the inducer and its applicability in whole wheat bread making that improves human health.






Similar content being viewed by others
References
Collin T, Gerday C, Feller G (2005) Xylanase, xylanase families and extremophilic xylanase. FEMS Microbiol Rev 29:3–23
Satyanarayana T, Sharma A, Mehta D, Puri AK, Kumar V, Nisha M, Joshi S (2012) In: Satyanarayana T, Johri BN, Anil Prakash (eds) Microorganisms in sustainable agriculture and biotechnology. Springer, Netherlands
Butt MS, Nadeem MT, Ahmad Z, Sultan MT (2008) Xylanases and their applications in baking industry. Food Technol Biotechnol 46:22–31
Hammer RJ (1995) In: Tucker G, Wood LFJ (eds) Enzymes in food processing, 2nd edn. Blackie Academic and Professional, Glasgow
Baillet E, Downey G, Tuohy M (2003) In: Courtin CM, Veraverbeke WS, Delcour JA (eds) Proceedings of the 3rd European Symposium on Enzymes in Grain Processing (ESEGP-3). Katholieke Universiteit Leuven, Leuven
Courtin CW, Delcour JA (2002) Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci 35:225–243
Gruppen H, Hamer RJ, Voragen AGJ (1992) Water-unextractable cell wall material from wheat flour. 2. Fractionation of alkali-extracted polymers and comparison with water-extractable arabinoxylans. J Cereal Sci 16:53–67
Wang M, van Vliet T, Hamer RJ (2004) Evidence that pentosans/xylanase affects the re-agglomeration of the gluten network. J Cereal Sci 39:341–349
Sorensen JF, Kragh KM, Sibbesen O, Delcour J, Goesaert H, Svensson B, Tahir TA, Brufau J, Perez-Vendrell AM, Bellincampi D, D’Ovidio R, Camardella L, Giovane A, Bonnin E, Juge N (2004) Potential role of glycosidase inhibitors in industrial biotechnological applications. Biochim Biophys Acta 1696:275–287
Qi Si J, Drost-Lustenberger C (2002) In: Whitehurst RJ, Law BA (eds) Enzymes in food technology. Sheffield Academic Press Ltd., Sheffield
Poutanen K (1997) Enzymes: an important tool in the improvement of the quality of cereal foods. Trends Food Sci Technol 8:300–306
Jin P, Bhattacharya SK, Williama CJ, Zhang H (1998) Effects of sulfide addition on copper inhibition in methanogenic systems. Water Res 32:977–988
Banik RM, Santhiagu A, Upadhyay SN (2007) Optimization of nutrients for gellan gum production by Sphingomonas paucimobilis ATCC-31461 in molasses based medium using response surface methodology. Bioresour Technol 98:792–797
Rao UJLM, Satyanarayana T (2007) Improving production of hyperthermostable and high maltose-forming a-amylase by an extreme thermophile Geobacillus thermoleovorans using response surface methodology and its applications. Bioresour Technol 98:345–352
Singh B, Satyanarayana T (2008) Phytase production by Sporotrichum thermophile in a cost-effective cane molasses medium in submerged fermentation and its application in bread. J Appl Microbiol 105:1858–1865
Biswas R, Sahai V, Mishra S, Bisaria VS (2010) Bioprocess strategies for enhanced production of xylanase by Melanocarpus albomyces IITD3A on agro-residual extract. J Biosci Bioeng 110:702–708
Kumar V, Satyanarayana T (2011) Applicability of thermo-alkali-stable and cellulase-free xylanase from a novel thermo-halo-alkaliphilic Bacillus halodurans in producing xylooligosaccharides. Biotechnol Lett 33:2279–2285
Kumar V, Satyanarayana T (2012) Thermo-alkali-stable xylanase of a novel polyextremophilic Bacillus halodurans TSEV1 and its application in biobleaching. Intr Biodet Biodegrad 75:138–145
Kumar V, Satyanarayana T (2013) Biochemical and thermodynamic characterization of thermo-alkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles 17:797–808
Archana A, Satyanarayana T (1997) Xylanase production by thermophilic Bacillus licheniformis A99 in solid state fermentation. Enzyme Microb Technol 21:12–17
Sahai V, Mishra S, Bisaria VS (2005) Cellulose-free nutrient medium for enhanced productivity and activity of cellulase-free enzymes. Indian Patent 1246/DEL/2005
Khasin A, Alchanati I, Shoham Y (1993) Purification and characterization of a thermostable xylanase from Bacillus stearothermophilus T-6. Appl Environ Microbiol 59:1725–1730
Sharma A, Adhikari S, Satyanarayana T (2007) Alkali-thermostable and cellulase free xylanase production by an extreme thermophile Geobacillus thermoleovorans. World J Microbiol Biotechnol 23:483–490
Techapun C, Poosaran N, Watanabe M, Sasaki K (2003) Thermostable and alkaline-tolerant microbial cellulase-free xylanases produced from agricultural wastes and the properties required for use in pulp bleaching bioprocesses: a review. Process Biochem 38:1327–1340
Anand A, Kumar V, Satyanarayana T (2013) Characteristics of thermostable endoxylanase and β-xylosidase of the extremely thermophilic bacterium Geobacillus thermodenitrificans TSAA1 and its applicability in generating xylooligosaccharides and xylose from agro-residues. Extremophiles 17:357–366
Hölker U, Höfer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186
Crueger W, Crueger A (2000) In: Crueger W, Crueger A (eds) Biotechnology, a textbook of industrial microbiology. Panima Publisher Corporation, New Delhi
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456
Ko CH, Lin ZP, Tu J, Tsai CH, Liu CC, Chen HT, Wng TP (2010) Xylanase production by Paenibacillus campinasensis BL11 and its pretreatment of hardwood kraft pulp bleaching. Intr Biodet Biodegrad 64:13–19
Horikoshi K (1991) In: Horikoshi K (ed) Microorganisms in alkaline environments. Kodansha Limited, Tokyo
Nampoothiri KM, Tomes GJ, Roopesh K, Szakacs G, Nagy V, Soccol CR, Pandey A (2004) Thermostable phytase production by Thermoascus aurantiacus in submerged fermentation. Appl Biochem Biotechnol 118:205–214
Price NC, Stevens L (1999) Fundamentals of enzymology: the cell and molecular biology of catalytic proteins. Oxford University Press, Oxford
Mamo G, Hatti-Kaul R, Mattiasson B (2006) A thermostable alkaline active endob-1-4-xylanase from Bacillus halodurans S7: purification and characterization. Enzyme Microb Technol 39:1492–1498
Shah AR, Madamwar D (2005) Xylanase production by a newly isolated Aspergillus foetidus strain and its characterization. Process Biochem 40:1763–1771
Honda H, Kudo T, Ikura Y, Horikoshi K (1985) Two types of xylanases of alkalophilic Bacillus sp. No. C-125. Can J Microbiol 31:538–542
Kumar V, Poonam S, Satyanarayana T (2013) Highly thermo-halo-alkali-stable β-1,4-endoxylanase from a novel polyextremophilic strain of Bacillus halodurans. Bioprocess Biosyst Eng 36:555–565
Wejse PL, Ingvorsen K, Mortensen KK (2003) Purification and characterization of two extremely halotolerant xylanases from a novel halophilic bacterium. Extremophiles 7:423–443
Giridhar PV, Chandra TS (2010) Production of novel halo-alkali-thermo-stable xylanase by a newly isolated moderately halophilic and alkali-tolerant Gracilibacillus sp. TSCPVG. Process Biochem 45:1730–1737
Hung KS, Liu SM, Fang TY, Tzou WS, Lin FP, Sun KH, Tang SJ (2011) Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Biotechnol Lett 33:1441–1447
Lai CS, Davis AB, Hoseney RC (1989) Functional effect of bran in bread-making. J Cereal Chem 66:217–219
Maat J, Roza M, Verbakel J, Stem H, Santos da Silva MJ, Bosse M, Egmond MR, Hagemans MLD, Gorcom RFM, Hessing JGM et al (1992) In: Visser J, Beldman G, Austers-vanSomeran MA, Voragen AGJ (eds) Xylan and xylanases. Elsevier, Amsterdam
Jiang Z, Li X, Yang S, Li L, Tan S (2005) Improvement of the breadmaking quality of wheat flour by the hyperthermophilic xylanase B from Thermotoga maritima. Food Res Int 38:37–43
Acknowledgments
The authors thank Mr. Vijay Kumar Gupta (Tushar Nutritive Food Industry, New Delhi) for extending help in assessing the applicability of endoxylanase in whole wheat bread making. VK is grateful to Indian Council of Medical Research, Govt. of India, New Delhi, for awarding senior research fellowship while carrying out this investigation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, V., Satyanarayana, T. Production of thermo-alkali-stable xylanase by a novel polyextremophilic Bacillus halodurans TSEV1 in cane molasses medium and its applicability in making whole wheat bread. Bioprocess Biosyst Eng 37, 1043–1053 (2014). https://doi.org/10.1007/s00449-013-1075-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1075-3