Abstract
In the boreal forests, feather mosses such as Hylocomium splendens and Pleurozium schreberi are colonized by cyanobacteria, which provide large amounts of nitrogen to forest ecosystems through nitrogen fixation. Although these feather mosses are also ubiquitous in subalpine forests of East Asia, little is known regarding their associated cyanobacteria and their ability to fix nitrogen. In this study, we investigated (1) whether cyanobacteria co-exist and fix nitrogen in the two species of feather mosses that cover the ground surface in a subalpine forest of Mt. Fuji, (2) whether cyanobacteria belonging to a common cluster with boreal forests are found in feather mosses in Mt. Fuji, and (3) whether moss-associated nitrogen fixation rates differed among moss growing substrates, canopy openness, and moss nitrogen concentrations in the same forest area. Our results showed that cyanobacteria colonized feather mosses in the subalpine forests of Mt. Fuji and acetylene reduction rates as an index of nitrogen fixation tended to be higher in H. splendens than in P. schreberi. Based on analysis of the nifH gene, 43 bacterial operational taxonomic units (OTUs) were identified, 28 of which represented cyanobacteria. Among the five clusters of cyanobacteria classified based on their nifH gene and identified in northern Europe, four (Nostoc cluster I, Nostoc cluster II, Stigonema cluster, and nifH2 cluster) were also found at Mt. Fuji. The acetylene reduction rate differed depending on the moss growing substrate and the total nitrogen concentration of moss shoots, and a strong negative correlation was observed with the total nitrogen concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Boreal forests are widely distributed in the Northern Hemisphere. Feather mosses cover the forest floor in these forests and have critical effects on soil moisture, temperature (Gornall et al. 2007), and chemistry (Cornelissen et al. 2007; Pacé et al. 2020). These feather mosses symbiotically associate with nitrogen-fixing cyanobacteria (DeLuca et al. 2002). The amount of nitrogen fixation by cyanobacteria associated with feather mosses is up to 7 kg N ha−1 year−1 (DeLuca et al. 2007; Lindo et al. 2013), which is comparable to or greater than the amount of nitrogen deposition in boreal forests (Gundale et al. 2011). Consequently, cyanobacteria on mosses play an important role in nitrogen cycling in boreal forests, where plant-available nitrogen is limited (Rousk et al. 2013).
Nitrogen fixation by free-living bacteria, including cyanobacteria, is often spatially heterogeneous, even within a single ecosystem component, and there are hotspots with particularly high fixation rates. In some cases, hotspots and non-hotspots are only a few centimeters apart (Reed et al. 2011). Moss-associated nitrogen fixation rates vary widely among and within forests (Deluca et al. 2002; Markham et al. 2009) and hotspots exist (Stuart et al. 2021b). Abiotic factors that affect the rate of nitrogen fixation by cyanobacteria on moss mats include water (Gundale et al. 2009, 2012b), temperature (Gundale et al. 2012a), light (Solheim et al. 2004; Gundale et al. 2012b), nitrogen availability (Zackrisson et al. 2004; Sorensen et al. 2012), and phosphorus availability (Zackrisson et al. 2004). Factors that cause variation in nitrogen fixation by cyanobacteria associated with feather mosses have been investigated in manipulative experiments. In addition, assessing the role of environmental factors in moss-associated nitrogen fixation by observational studies using natural gradients of nutrients or seasonal variations can validate the results of manipulation experiments in a real environment (Renaudin et al. 2022). However, few observational studies have addressed the environmental factors controlling spatial variation in nitrogen fixation within a single forest stand. The influence of the moss-growing substrate on moss-associated nitrogen fixation has also been overlooked. Decaying fallen logs promote seedling establishment in boreal and subalpine forests and are ecologically important in forest regeneration (Simard et al. 1998; Narukawa et al. 2003). Fallen logs can also be active sites for N2 fixers, mainly methanotrophs, and the acetylene reduction rate varies depending on the extent of their decay (Mäkipää et al. 2018). However, little is known about differences in moss-associated nitrogen fixation on fallen logs and on the ground.
Moss-associated nitrogen fixation is largely controlled by the taxonomic identity of mosses (Stuart et al. 2021b). Because the two feather moss species, Hylocomium splendens and Pleurozium schreberi, are ubiquitous in boreal forests, many studies have evaluated the relationship between nitrogen fixation and biotic factors in their communities. Both H. splendens and P. schreberi are typical boreal forest feather mosses, but H. splendens prefers more productive sites, whereas P. schreberi is distributed over a wider range of ecological conditions (Påhlsson 1994). Both H. splendens and P. schreberi decrease the nitrogen fixation rate per unit area of moss as N availability increases, but nitrogen fixation associated with H. splendens is tolerant to low amounts of N supply (5 kg N ha−1 year−1) (Zackrisson et al. 2009). Also, differences in hydration rates brought about by morphological characteristics of mosses in both species control cyanobacterial colonization and nitrogen fixation (Liu and Rousk 2022). H. splendens and P. schreberi are colonized by epiphytic cyanobacteria of the genera Calothrix, Cylindrospermum, Nostoc, and Stigonema (Gentili et al. 2005; Houle et al. 2006; Zackrisson et al. 2009; Ininbergs et al. 2011). A phylogenetic analysis based on the nitrogenase gene (nifH) identified five clusters of cyanobacteria that grow on H. splendens and P. schreberi in Swedish forests (Nostoc cluster I, Nostoc cluster II, Stigonema cluster, nifH2 cluster, and mixed cluster). That same study found evidence of a host-specific relationship between feather moss species and cyanobacterial communities (Ininbergs et al. 2011). Furthermore, changes in the expression of nifH in each of these clusters result in seasonal variations in nitrogen fixation associated with H. splendens and P. schreberi (Warshan et al. 2016). In Alaskan mosses (including H. splendens and P. schreberi), the bacterial community composition, including cyanobacteria, is also strongly host-specific (Holland-Moritz et al. 2018, 2021). There is a correlation between the phylogenetic distance of the host mosses and differences in the composition of the moss microbiome, and some putative N2-fixing bacteria other than cyanobacteria have their relative abundance correlated with the rate of nitrogen fixation associated with mosses (Holland-Moritz et al. 2021).
Coniferous forests consisting of Abies spp. and Picea spp., as well as boreal forests, are ubiquitous in the subalpine zone of the high mountains of East Asia; Tsuga spp. are also present (Franklin et al. 1979; Ohsawa 1990, 1993). In subalpine coniferous forests at altitudes of 1500–2500 m in central Honshu, Japan, communities of mosses common to boreal forests (e.g., H. splendens and P. schreberi) develop on the forest floor. In these settings, mosses often cover the entire forest floor, and the two aforementioned species constitute > 97% of the moss community (Nakamura 1984). By supporting seedling establishment, moss communities play an important role in the maintenance and regeneration of subalpine coniferous forests (Franklin et al. 1979; Nakamura 1987, 1992; Sugita and Nagaike 2005; Katsumata et al. 2008); a close relationship between mosses and nitrogen-fixing cyanobacterial communities might contribute to the maintenance and regeneration of these forests. However, neither nitrogen fixation by cyanobacteria, nor the community composition of cyanobacteria associated with the feather mosses H. splendens and P. schreberi in coniferous forests in East Asia (e.g., subalpine forests in Japan), has been extensively investigated.
This study was conducted in Japanese subalpine forests, especially in the subalpine forests of Mt. Fuji, where a homogeneous moss mat is spreading on the forest floor, and evaluated three hypotheses: (1) nitrogen fixation associated with H. splendens and P. schreberi occurs in the subalpine forests of Mt. Fuji, which is spatially distant from boreal forests in northern Europe and North America; (2) cyanobacteria in the same clusters as those in boreal forests are present in subalpine forests; and (3) moss-related nitrogen fixation varies with moss nitrogen demand, light conditions, and growth substrate, even within the same forest stand.
Materials and methods
Study site
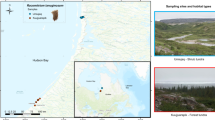
The study site is a subalpine coniferous forest (35°23.9′ N, 138°42.0′ E, 2000 m above sea level) located on the north slope of Mt. Fuji. The study site is a natural forest located within a special zone (nature reserve area) of Fuji-Hakone-Izu National Park, where forest management and grazing are not conducted. The annual mean temperature of the study area is 4.4 °C, according to data from Japan Meteorological Agency stations located at the summit of Mt. Fuji and at Lake Kawaguchi. The mean annual precipitation near the study site is 2500 mm (Yamamoto 1971).
The surveyed forest is a climax forest (basal area 63.3 m2 ha−1), dominated by Tsuga diversifolia (relative basal area 98%) but interspersed with Abies veitchii, Abies mariesii, and Rhododendron brachycarpum. The forest floor is covered with well-mixed moss communities, dominated by H. splendens and P. schreberi (86% and 71% of vegetation cover, respectively), with a small proportion of Dicranum majus (5%).
Sampling
Sampling was conducted on September 16, 2016. Ten shoots each of H. splendens and P. schreberi were collected from 10 fallen logs and from the ground surface within 1 m of each log in an approximately 1-ha area of the study site; thus, 400 moss shoots were collected in total (2 moss species × 2 habitats [log and ground] × 10 sampling locations × 10 shoots). The collected moss shoots were stored at 5–8 °C; nitrogen fixation rates were measured within 12 days.
Measurement of acetylene reduction rate
To estimate the nitrogen fixation rates associated with the sampled mosses, acetylene reduction rates were measured according to the method of Hardy et al. (1968). Five shoots of H. splendens and 10 shoots of P. schreberi, sprayed with purified water at each sampling location and habitat, were enclosed in a 27 mL glass vial with a septum (Ininbergs et al. 2011). With the exception of the rhizoid, the entire shoot was used. The vials containing the moss shoots were incubated at 23 °C for 2 days under a light:dark cycle of 16 h light (photosynthetic photon flux density: 44 μmol m−2 s−1):8 h dark. After incubation, vials were ventilated and plugged. Next, 2.7 mL of air was removed from each vial and the same volume of acetylene gas was added. The samples were incubated under the same conditions for 1 day. After incubation, 1 mL of the gas phase in the vial was collected and the amount of ethylene produced was measured using a gas chromatograph (G-3500; Hitachi, Tokyo, Japan). The column packing material of the gas chromatograph was activated alumina, and a flame ionization detector was used. After the rate of cyanobacterial colonization had been measured, the shoots were freeze-dried, and their dry weights were determined. The amounts of ethylene produced by the natural reduction of acetylene (production of ethylene in the absence of a sample) and by the moss itself (production of ethylene by a sample without acetylene addition) were measured as controls; the total amount of ethylene in the samples minus the total amount of ethylene in the controls was regarded as the true amount of ethylene produced by the samples. Total ethylene production in the controls was calculated per vial. The acetylene reduction rate per shoot dry weight was calculated from the true amount of ethylene produced by the samples as an index of nitrogen fixation.
Measurement of cyanobacterial colonization rate
After the nitrogen fixation rate had been measured, 10 shoots of each sample were observed under an epifluorescence microscope (SZX12; Olympus, Tokyo, Japan) fitted with a green excitation filter. The cyanobacterial colonization rate was calculated for each sample as the proportion of shoots containing cyanobacteria. For H. splendens, the cyanobacterial colonization rate was calculated by combining the five shoots used in the nitrogen fixation rate measurements with five other shoots collected from the same sampling location and habitat.
Environmental factors that correlate with the rate of acetylene reduction and cyanobacterial colonization
Eco-physiological factors that correlate with acetylene reduction and cyanobacterial colonization on moss mats were assessed by measuring total nitrogen concentration in moss shoots and canopy openness at each sampling location for both H. splendens and P. schreberi. Canopy openness was determined in panoramic photographs that had been acquired at each sampling location using a 360° camera (THETA S; Richo, Tokyo, Japan); the openness was calculated using RGBFisheye (Ishida 2004). Samples used to determine the rates of nitrogen fixation and cyanobacterial colonization were homogenized at 1800 rpm for 90 s using a mixer mill (MM400; Verder Scientific, Haan, Germany). The total nitrogen concentrations in the samples were analyzed using an elemental analyzer (SUMIGRAPH NCH-22; Sumika Chemical Analysis Service, Tokyo, Japan).
Examination of cyanobacteria community composition based on nifH gene analysis
The community composition of cyanobacteria living on each sample of H. splendens and P. schreberi was investigated via phylogenetic analysis of the nifH gene. DNA was extracted from 10 mg of the above-described homogenized moss leaf samples using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany).
A tenfold dilution of the DNA extract was used as the template; the nifH region of cyanobacterial DNA was polymerase chain reaction (PCR)-amplified using the cyanobacteria-specific primers CNF/CNR (Olson et al. 1998), together with the KAPA Taq EXtra PCR kit (KAPA Biosystems, Boston, MA, USA). The PCR solution was prepared in accordance with the kit manufacturer’s protocol. Amplification was performed as follows: initial denaturation at 95 °C for 3 min; 40 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min; and final extension at 72 °C for 7 min.
PCR products were integrated into the pMD20 T-vector (Takara Bio, Shiga, Japan) using the DNA Mighty Mix ligation kit (Takara Bio), then transferred into Escherichia coli HST08 competent cells (Takara Bio) in accordance with the manufacturer’s instructions. The subcloned fragments were amplified from plasmid DNA in 12 positive clones using the primers U19 and M13R. The PCR products were purified using Illustra ExoProStar (GE Healthcare, Buckinghamshire, UK) and sequenced by Macrogen Japan (Tokyo, Japan) using primer SP6.
The obtained 480 sequences were clustered using CD-HIT Suite (http://weizhongli-lab.org/cdhit_suite/cgi-bin/index.cgi?cmd=cd-hit-est; Huang et al. 2010) and classified into operational taxonomic units (OTUs), using 97% sequence homology as the threshold. Representative sequences of each OTU were searched against the database of bacteria with high homology via BLAST (https://www.ncbi.nlm.nih.gov/) and divided into cyanobacteria or non-cyanobacteria according to the most homologous reference sequence. OTU sequences identified as cyanobacteria were combined with reference sequences that belonged to Nostoc cluster I, Nostoc cluster II, Stigonema cluster, nifH2 cluster, and mixed cluster (Ininbergs et al. 2011); multiple alignments were generated using MAFFT version 7 (https://mafft.cbrc.jp/alignment/server/; Kuraku et al. 2013; Katoh et al. 2019). A maximum likelihood phylogenetic tree was created using the IQ-TREE web server (http://iqtree.cibiv.univie.ac.at/; Nguyen et al. 2015; Trifinopoulos et al. 2016; Kalyaanamoorthy et al. 2017) with Trichodesmium erythraeum (Accession number L00689) as the outgroup. Bootstrap values were calculated from 1000 iterations of bootstrap resampling via UFBoot (Hoang et al. 2018). Reference sequence information was obtained from GenBank and from Integrated Microbial Genomes (http://img.jgi.doe.gov/). Sequences of each OTU are registered in the DDBJ database (accession numbers LC716857–LC716899).
Statistical analysis
Differences in log-transformed acetylene reduction rates by moss species and moss growth substrate (fallen logs/ground) were examined by repeated measures analysis of variance (ANOVA) and post hoc analysis by Tukey's honestly significant difference test. The normality of the data was confirmed by the Shapiro–Wilk test and the homoscedasticity of each group by the Bartlett test. Multiple comparisons of cyanobacterial colonization rates were conducted by Bonferroni-corrected Wilcoxon signed-rank test between moss species and growth substrates. A simple linear regression analysis was performed with log-transformed acetylene reduction rate as the objective variable and cyanobacterial colonization rate as the explanatory variable.
A generalized linear model (GLM) was used to analyze environmental factors correlated with acetylene reduction rates and cyanobacterial colonization rates for each moss species. Growth substrate (fallen logs/ground), canopy openness of the sampling location, and total nitrogen concentration in moss shoots served as explanatory variables. In GLMs, a gamma error distribution and log link function were used for the acetylene reduction rate, while a pseudo-binomial error distribution and logit link function were used for the colonization rate. Hypothesis tests of the data fitted to the GLM were conducted using an analysis of deviance; F tests were employed to extract factors that correlate with the rates of acetylene reduction and cyanobacterial colonization.
A permutational multivariate analysis of variance (PERMANOVA) was conducted to evaluate differences in the cyanobacterial community composition according to moss species, growth habitat, and sampling location. The β-diversity index, which represents the difference in cyanobacterial community composition among samples, was determined using both the Bray–Curtis index, calculated using the number of confirmed cyanobacterial OTU sequences in each sample, and the Jaccard index, calculated using binarized data that represent the presence/absence of each OTU. The results were analyzed via PERMANOVA with 10,000 permutation simulations. Data regarding non-cyanobacterial OTUs were excluded, as were data from sampling locations that contained only non-cyanobacterial OTUs.
R software, version 3.6.3 (R Core Team 2020), was used for all statistical analyses; the package “car” (Fox and Weisberg 2019) was used for analysis of deviance and the package “vegan” (Oksanen et al. 2020) was used for PERMANOVA.
Results
Acetylene reduction rates and colonization rates in moss mats of H. splendens and P. schreberi
The acetylene reduction rate (mean ± standard deviation) of H. splendens was 0.68 ± 0.72 μmol g−1 day−1 on fallen logs and 0.27 ± 0.39 μmol g−1 day−1 on the ground surface; the corresponding values for P. schreberi were 0.20 ± 0.31 μmol g−1 day−1 and 0.10 ± 0.14 μmol g−1 day−1.
There was strong evidence for differences in log-transformed acetylene reduction rates between H. splendens and P. schreberi (ANOVA: F = 11.0, P = 0.002), and a trend towards differences in log-transformed acetylene reduction rates associated with mosses collected from fallen logs and the ground surface (F = 3.9, P = 0.053). The acetylene reduction rate associated with H. splendens was higher than P. schreberi (Fig. 1). The acetylene reduction rate associated with moss samples collected from fallen logs tended to be higher than that of samples collected from the ground surface (Fig. 1). The cyanobacterial colonization rate of mosses was higher on H. splendens than on P. schreberi and on fallen logs than on the ground surface (Fig. 2). The log-transformed acetylene reduction rate associated with each moss species in each growth substrate (fallen logs/ground) was strongly positively correlated with the cyanobacterial colonization rate (Fig. 3).
Acetylene reduction rates associated with H. splendens and P. schreberi (n = 10). Horizontal line, median; lower end of box, first quartile (25%); upper end of box, third quartile (75%). Ends of the whiskers represent the maximum and minimum values within 1.5 times the length of the box. Different letters indicate significant differences between log-transformed values (post hoc Tukey’s honestly significant difference test, P < 0.05)
Cyanobacterial colonization rates of H. splendens and P. schreberi (n = 10). In the boxplot, the horizontal line shows the median, the lower end of the box shows the first quartile (25%), and the upper end of the box shows the third quartile (75%). The ends of the whiskers are the maximum and minimum values within 1.5 times the length of the box. Different letters in the figure indicate significant differences (Bonferroni-corrected Wilcoxon signed-rank test, P < 0.01)
Relationship between cyanobacterial colonization rate and the logarithmic conversion value of the acetylene reduction rate (n = 10). Figures on the left show the relationship in H. splendens, while figures on the right show the relationship in P. schreberi. The upper figures show the relationship in mosses on fallen logs, while the lower figures show the relationship in mosses on the ground. The colonization rate was calculated as the proportion of shoots with cyanobacterial colonization among the 10 included shoots
Environmental factors that correlate with the rate of acetylene reduction and cyanobacterial colonization
There was moderate evidence that the total nitrogen concentration in moss shoots was correlated with the acetylene reduction rates associated with both H. splendens and P. schreberi (analysis of deviance, type II test: F = 8.5 and 8.2, respectively; P < 0.05) and it correlated negatively with the acetylene reduction rate (Table 1). For H. splendens, there was moderate evidence that the acetylene reduction rate varied with moss growth substrate (analysis of deviance, type II test: F = 5.0, P = 0.041); H. splendens collected from fallen logs had higher acetylene reduction rate than those collected from the ground surface (Table 1). For both H. splendens and P. schreberi, there was no evidence that the acetylene reduction rates were not correlated with openness (analysis of deviance, type II test: F = 1.2 and 0.3, respectively; P > 0.1).
There was strong evidence that the total nitrogen concentration in moss shoots of H. splendens and P. schreberi was correlated with the cyanobacterial colonization rate (analysis of deviance, type II test: F = 9.5 and 10.8, respectively; P < 0.01); it was negatively correlated with the cyanobacterial colonization rate (Table 2). For H. splendens, there was strong evidence that canopy openness was correlated with cyanobacterial colonization (analysis of deviance, type II test: F = 9.1, P = 0.008); it was positively correlated with the cyanobacterial colonization rate (Table 2). By contrast, for P. schreberi, there was no evidence that openness was correlated with the colonization rate, but the colonization rate tended to differ depending on the growth substrate (analysis of deviance, type II test: F = 4.2, P = 0.057), with samples from fallen logs tending to have higher colonization rates than those from the ground surface (Table 2).
Community composition of cyanobacteria living on H. splendens and P. schreberi
Of the 43 OTUs, 28 were classified as cyanobacteria and the remaining 15 were classified as non-cyanobacteria, based on the BLAST results. In the phylogenetic tree of cyanobacterial OTUs, Nostoc cluster I, identified by Ininbergs et al. (2011), was not monophyletic; it was located in the same clade as the Stigonema cluster (Fig. 4a, b). Of the 28 cyanobacteria OTUs, 10 were classified as Nostoc cluster I and Stigonema cluster, 3 were classified as Nostoc cluster II, and 15 were classified as nifH2 cluster (Fig. b–d). In the nifH2 cluster, 12 OTUs were classified into a subcluster that we designated as the “Fuji subcluster” (Fig. 4d). Of the 28 cyanobacterial OTUs, 23 were found on H. splendens, 19 were found on P. schreberi, and 14 were found on both species (Fig. 4b–d).
Phylogenetic tree of a all clusters, b Nostoc cluster I and Stigonema cluster, c Nostoc cluster II, and d nifH2 cluster based on the partial nifH sequence of cyanobacteria found on H. splendens and P. schreberi, determined using the maximum likelihood method. The numbers represent ultrafast bootstrap values. Reference sequences were obtained from GenBank and Integrated Microbial Genomes. Sequences labeled cyano_OTU are from this study; other sequences are from the phylogenetic tree in the work by Ininbergs et al. (2011). White and black circles indicate OTUs detected in H. splendens and P. schreberi, respectively
Among the non-cyanobacterial OTUs, the most homologous (> 90%) bacterial sequences in BLAST were Bradyrhizobium (4 OTUs), Methylocella (2 OTUs), Methyloferula (2 OTUs), and unknown (7 OTUs).
When the Bray–Curtis index was used as the β-diversity index, PERMANOVA identified moss species and sampling location as factors that significantly affected the community composition of cyanobacterial OTUs; however, when the Jaccard index was used as the β-diversity index, only the sampling location was significant (Table 3).
Discussion
Acetylene reduction rate in subalpine moss carpets of H. splendens and P. schreberi
In our study, the acetylene reduction rate of H. splendens tended to be higher than that of P. schreberi. Zackrisson et al. (2009) reported that H. splendens had a lower sensitivity of associated acetylene reduction rate per unit area to nitrogen deposition compared with P. schreberi; when N availability was low, the acetylene reduction rate was inferior to P. schreberi, but when N availability was high, the acetylene reduction rate was slightly higher than P. schreberi. Jean et al. (2020) reported that H. splendens had a higher rate of nitrogen fixation per dry weight of moss and a higher abundance of genus Nostoc compared to P. schreberi. They speculated that P. schreberi had a lower nitrogen requirement than H. splendens since P. schreberi could tolerate a wider range of habitats and lower nutrient availability. In the pine forests at the northern foot of Mt. Fuji (8 km from the study site), nitrogen deposition is approximately 10 kg N ha−1 year−1, which is regarded as nitrogen saturation in forest ecosystems (Matsumoto et al. 2020). Furthermore, the study area is surrounded by a busy mountain road (Mt. Fuji Toll Road Fuji Subaru Line), and air pollution by NO and NO2 has been confirmed in the forest near the road (Wada et al. 2010). P. schreberi around high-traffic roads have lower acetylene reduction rates than P. schreberi around low-traffic roads (Ackermann et al. 2012). In addition, cyanobacterial colonization of P. schreberi in this study tended to be lower than that of H. splendens, suggesting P. schreberi may not actively attract cyanobacteria due to increased N availability caused by air pollution around Mt. Fuji subalpine forest, and as a result, the acetylene reduction rate may be lower than that of H. splendens. However, the nitrogen fixation rate of H. splendens and P. schreberi is seasonally and annually variable (Warshan et al. 2016; Jean et al. 2018), and our data were obtained only in September 2016.
The dry weight (mean ± standard deviation) per unit area of mosses in the study area was 141 ± 69 g m−2 for H. splendens and 90 ± 28 g m−2 for P. schreberi (Nakamura 1984). The mean (± standard deviation) acetylene reduction rates per unit land area calculated using these values were 95.3 ± 101.5 μmol m−2 day−1 on fallen logs and 38.2 ± 54.6 μmol m−2 day−1 on the ground surface for H. splendens; corresponding values for P. schreberi were 17.9 ± 27.5 μmol m−2 day−1 and 9.4 ± 12.78 μmol m−2 day−1, respectively. Using a moss growth period of 200 days and a conversion ratio of 3 mol acetylene molecules to 1 mol nitrogen molecules (DeLuca et al. 2002; Zackrisson et al. 2009), the nitrogen fixation associated with H. splendens and P. schreberi at the surface was calculated to be 0.89 ± 1.3 kg N ha−1 year−1. This value is one-eighth to one-half of the nitrogen fixation reported in late successional and less susceptible to nitrogen deposition boreal forests in Northern Europe and in Alaskan boreal spruce forest (Zackrisson et al. 2004, 2009; DeLuca et al. 2007; Jean et al. 2018). There is a clear latitudinal gradient in nitrogen fixation per area and per moss dry weight associated with H. splendens and P. schreberi from northern to southern Fennoscandia, and it is speculated that nitrogen fixation is greatly limited by anthropogenic nitrogen deposition in boreal forests of southern Fennoscandia (Zackrisson et al. 2009; Salemaa et al. 2019). As mentioned above, there is a busy mountain road around the study site on Mt. Fuji and high nitrogen deposition near the study site has been reported; such high anthropogenic nitrogen supply may have resulted in inferior nitrogen fixation associated with feather moss on the ground surface in the subalpine forest of Mt. Fuji compared to the boreal forest in the northern Fennoscandian region. Light availability on the forest floor may also explain differences in nitrogen fixation on the moss forest floor in subalpine forests of Mt. Fuji and boreal forests of northern Fennoscandia and Alaska. The basal area of the study site is up to threefold that of boreal forests with feathermoss-associated nitrogen fixation (DeLuca et al. 2007; Melvin et al. 2015; Jean et al. 2018), and canopy coverage, calculated from the openness of the sampled sites, is as high as in Alaskan paper birch forests with lower nitrogen fixation rates associated with feather mosses (Jean et al. 2018). Canopy cover is negatively correlated with the abundance of Nostocaceae in feather mosses, resulting in reduced nitrogen fixation per moss dry weight (Jean et al. 2020). Therefore, cyanobacterial abundance and nitrogen fixation may be lower in the subalpine forests of Mt. Fuji due to low light availability caused by high forest canopy cover compared to boreal forests in northern Fennoscandia and Alaska. However, the nitrogen fixation rate was based on acetylene reduction measured in the laboratory 12 days after sample collection, values for moss biomass in 1984 were used, and the conversion ratio between acetylene and nitrogen molecules can vary with moss species and time (Saiz et al. 2019).
There was a strong positive correlation between the acetylene reduction rate and the cyanobacterial colonization rate in both H. splendens and P. schreberi, consistent with findings in previous studies (DeLuca et al. 2007; Stuart et al. 2021a; Liu and Rousk 2022; Renaudin et al. 2022). While positive correlations between nitrogen fixation and relative abundances of bacteria other than cyanobacteria have been reported for some moss species (Holland-Moritz et al. 2021), the strong correlation between the acetylene reduction rate and the rate of cyanobacterial colonization in this study suggests that cyanobacteria contribute to nitrogen fixation on moss carpets in subalpine forest of Mt. Fuji. However, because nitrogen-fixing bacteria other than cyanobacteria carrying the nifH gene were also identified, and nitrogen-fixing activity was observed even in samples with low cyanobacterial colonization rates (10%), the possibility that nitrogen-fixing bacteria other than cyanobacteria contributed to the acetylene reduction rates cannot be excluded.
Environmental factors that correlate with the rate of acetylene reduction and cyanobacterial colonization
In this study, among the analyzed factors (i.e., growth substrate [fallen logs or ground surface], openness, and total nitrogen concentration in moss shoots), the strongest correlation with the acetylene reduction rate and colonization rate was mediated by the total nitrogen concentration in moss shoots, which was negatively correlated with the acetylene reduction rate and colonization rate in both moss species. Mosses in nitrogen-poor environments release chemical signals that promote the differentiation of cyanobacteria into hormogonia, a motile form that enhances the colonization of moss shoots (Bay et al. 2013). This may explain our finding of a negative correlation between the cyanobacterial colonization rate and the total nitrogen concentration in moss shoots. A study of seven forest sites in Finland identified positive correlations between nitrogen fixation rates per moss dry weight and the C/N ratios of moss shoots of H. splendens and P. schreberi (Leppänen et al. 2013). Because our results were obtained from samples in a 1-ha forest area, the negative correlation between the acetylene reduction rate and the total nitrogen concentration in moss shoots strongly suggests that cyanobacterial nitrogen fixation activity has high sensitivity to the nitrogen requirement of the host moss.
Light availability was positively correlated with the abundance of Nostocaceae associated with feather mosses (Jean et al. 2020), and the relationship between the cyanobacterial colonization rate and the canopy openness in H. splendens in this study is consistent with a prior report. By contrast, there was no correlation between the acetylene reduction rate and openness. Jean et al. (2020) reported that in both H. splendens and P. schreberi, canopy cover has a negative effect on moss-related nitrogen fixation directly or through relative abundance of Nostocaceae. In manipulation experiments, light availability was positively correlated with nitrogen fixation except at temperatures exceeding 30 °C (Gundale et al. 2012a; Sorensen et al. 2012). This discrepancy may be due to the fact that the mosses were incubated under the same light conditions in the laboratory during the acetylene reduction assay. It has been suggested that the tree canopy changes not only light availability but also the amount and form of nitrogen in rainfall, affecting nitrogen fixation associated with feathermoss on the forest floor (Salemaa et al. 2019). Because tree canopy reduces total N deposition and increases the proportion of dissolved organic nitrogen (DON) in through fall, sites with greater openness have more inorganic nitrogen, which is likely to negatively affect nitrogen fixation associated with feather moss, compared to sites with less openness (Salemaa et al. 2019).
In the GLM, acetylene reduction rates associated with H. splendens tended to be higher in samples collected from fallen logs than from the ground surface. In addition, although not significant by analysis of deviance, multiple comparisons showed that the cyanobacterial colonization rates of H. splendens and P. schreberi tended to be higher in samples collected from fallen logs than from the ground surface. The reasons for these findings are unclear, but microenvironmental differences such as light availability, leaf litter accumulation (Jean et al. 2020), and nutrient availability may have contributed. H. splendens and P. schreberi appear when logs are considerably decomposed (Nakamura 1987). Fallen logs, as coarse woody debris, can be a source of nutrients such as phosphorus as they decompose (Laiho and Prescott 2004; Brais et al. 2006). Cases of phosphorus promoting cyanobacterial biomass (Renaudin et al. 2022) and acetylene reduction (Zackrisson et al. 2004) have been reported. This result may be due to the greater P supply from decayed fallen logs than from the ground surface.
Community composition of cyanobacteria on H. splendens and P. schreberi
Among the five cyanobacterial clusters identified by Ininbergs et al. (2011), OTUs that belonged to four clusters (all but the mixed cluster) were detected in this study. To our knowledge, this is the first report that major cyanobacterial clusters common to the boreal forests of Northern Europe are also present in Japanese subalpine forests. These four cyanobacterial clusters are widely distributed in the Northern Hemisphere, along with H. splendens and P. schreberi. However, in the present study, the largest number of OTUs belonged to the nifH2 cluster; 12 of 15 OTUs in that cluster belonged to the “Fuji subcluster,” which is rare in the boreal forests of Northern Europe (Ininbergs et al. 2011). These results suggest global differences in terms of cyanobacterial communities between boreal forests and subalpine forests in the temperate zone.
PERMANOVA showed no effect of moss species on cyanobacterial community composition, according to presence/absence data (i.e., using the Jaccard index); however, a difference was detected when abundance data (i.e., using the Bray–Curtis index) were used. Therefore, although the basic cyanobacterial community composition was similar for the two moss species, there may be subtle differences in the composition preferred by each moss species. Differences in host moss phylogenetic distance and moss microbiome composition are correlated (Holland-Moritz et al. 2021). The two mosses in this study belong to the same family; they have a close phylogenetic relationship. Notably, we found that half of the identified OTUs of cyanobacteria were present in both H. splendens and P. schreberi, suggesting that the two mosses are intermixed and thus share some strains of cyanobacteria for nitrogen fixation.
In conclusion, this study revealed the following: (1) In a subalpine forest on Mt. Fuji, cyanobacteria colonized and fixed nitrogen in feather mosses, and H. splendens was superior to P. schreberi in acetylene reduction rate in September. (2) Analysis of nifH of cyanobacteria on feather mosses in a subalpine forest of Mt. Fuji revealed a community composed of four cyanobacterial clusters that are common to boreal forests. (3) Even within a seemingly homogeneous forest stand, the acetylene reduction rate associated with feather mosses differed depending on the growth substrate and the total nitrogen concentration of the mosses, and had a particularly strong relationship with the latter.
To our knowledge, this is the first phylogenetic analysis of cyanobacteria on feather mosses, and specifically on H. splendens and P. schreberi, in East Asia. The data obtained from this subalpine forest, which is spatially remote from boreal forests, will contribute to a better understanding of consistency of responses to environmental factors and cyanobacterial community structure in moss–cyanobacteria associations.
Availability of data and materials
The datasets used in this study are available from the corresponding author on reasonable request. Sequences of each OTU are registered in the DDBJ database (accession numbers LC716857–LC716899).
Code availability
Not applicable.
References
Ackermann K, Zackrisson O, Rousk J, Jones DL, DeLuca H (2012) N2 fixation in feather mosses is a sensitive indicator of N deposition in boreal forests. Ecosystems 15:986–998. https://doi.org/10.1007/s10021-012-9562-y
Bay G, Nahar N, Oubre M, Whitehouse MJ, Wardle DA, Zackrisson O, Nilsson MC, Rasmussen U (2013) Boreal feather mosses secrete chemical signals to gain nitrogen. New Phytol 200:54–60. https://doi.org/10.1111/nph.12403
Brais S, Paré D, Lierman C (2006) Tree bole mineralization rates of four species of the Canadian eastern boreal forest: implications for nutrient dynamics following stand-replacing disturbances. Can J for Res 36:2331–2340. https://doi.org/10.1139/x06-136
Cornelissen JH, Lang SI, Soudzilovskaia NA, During HJ (2007) Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Ann Bot 99:987–1001. https://doi.org/10.1093/aob/mcm030
DeLuca TH, Zackrisson O, Nilsson MC, Sellstedt A (2002) Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419:917–920. https://doi.org/10.1038/nature01051
DeLuca TH, Zackrisson O, Gentili F, Sellstedt A, Nilsson MC (2007) Ecosystem controls on nitrogen fixation in boreal feather moss communities. Oecologia 152:121–130. https://doi.org/10.1007/s00442-006-0626-6
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks CA. https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Franklin JF, Maeda T, Ohsumi Y, Matsui M, Yagi H, Hawk GM (1979) Subalpine coniferous forests of central Honshu, Japan. Ecol Monogr 49:311–334. https://doi.org/10.2307/1942487
Gentili F, Nilsson MC, Zackrisson O, DeLuca TH, Sellstedt A (2005) Physiological and molecular diversity of feather moss associative N2-fixing cyanobacteria. J Exp Bot 56:3121–3127. https://doi.org/10.1093/jxb/eri309
Gornall JL, Jónsdóttir IS, Woodin SJ, Van der Wal R (2007) Arctic mosses govern below-ground environment and ecosystem processes. Oecologia 153:931–941. https://doi.org/10.1007/s00442-007-0785-0
Gundale MJ, Gustafsson H, Nilsson MC (2009) The sensitivity of nitrogen fixation by a feathermoss–cyanobacteria association to litter and moisture variability in young and old boreal forests. Can J for Res 39:2542–2549. https://doi.org/10.1139/X09-160
Gundale MJ, DeLuca TH, Nordin A (2011) Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Glob Change Biol 17:2743–2753. https://doi.org/10.1111/j.1365-2486.2011.02407.x
Gundale MJ, Nilsson M, Bansal S, Jäderlund A (2012a) The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol 194:453–463. https://doi.org/10.1111/j.1469-8137.2012.04071.x
Gundale MJ, Wardle DA, Nilsson MC (2012b) The effect of altered macroclimate on N-fixation by boreal feather mosses. Biol Lett 8:805–808. https://doi.org/10.1098/rsbl.2012.0429
Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol 43:1185–1207. https://doi.org/10.1104/pp.43.8.1185
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522. https://doi.org/10.1093/molbev/msx281
Holland-Moritz H, Stuart J, Lewis LR, Miller S, Mack MC, McDaniel SF, Fierer N (2018) Novel bacterial lineages associated with boreal moss species. Environ Microbiol 20:2625–2638. https://doi.org/10.1111/1462-2920.14288
Holland-Moritz H, Stuart JEM, Lewis LR, Miller SN, Mack MC, Ponciano JM, McDaniel SF, Fierer N (2021) The bacterial communities of Alaskan mosses and their contributions to N2-fixation. Microbiome 9:53. https://doi.org/10.1186/s40168-021-01001-4
Houle D, Bilodeau Gauthier S, Paquet S, Planas D, Warren A (2006) Identification of two genera of N2-fixing cyanobacteria growing on three feather moss species in boreal forests of Quebec, Canada. Botany 84:1025–1029. https://doi.org/10.1139/b06-059
Huang Y, Niu B, Gao Y, Fu L, Li W (2010) CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics 26:680–682. https://doi.org/10.1093/bioinformatics/btq003
Ininbergs K, Bay G, Rasmussen U, Wardle DA, Nilsson MC (2011) Composition and diversity of nifH genes of nitrogen-fixing cyanobacteria associated with boreal forest feather mosses. New Phytol 192:507–517. https://doi.org/10.1111/j.1469-8137.2011.03809.x
Ishida M (2004) Automatic thresholding for digital hemispherical photography. Can J for Res 34:2208–2216. https://doi.org/10.1139/x04-103
Jean M, Mack MC, Johnstone JF (2018) Spatial and temporal variation in moss-associated dinitrogen fixation in coniferous-and deciduous-dominated Alaskan boreal forests. Plant Ecol 219:837–851. https://doi.org/10.1007/s11258-018-0838-y
Jean M, Holland-Moritz H, Melvin AM, Johnstone JF, Mack MC (2020) Experimental assessment of tree canopy and leaf litter controls on the microbiome and nitrogen fixation rates of two boreal mosses. New Phytol 227:1335–1349. https://doi.org/10.1111/nph.16611
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Katsumata N, Okitsu S, Minami Y (2008) Influence of feather mossmat on the occurrence and growth of Abies veitchii seedlings in subalpine forest, Mt. Fuji, Japan. Lindbergia 33:50–57 http://www.jstor.org/stable/27809542
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform 20:1160–1166. https://doi.org/10.1093/bib/bbx108
Kuraku S, Zmasek CM, Nishimura O, Katoh K (2013) aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucl Acids Res 41:W22–W28. https://doi.org/10.1093/nar/gkt389
Laiho R, Prescott CE (2004) Decay and nutrient dynamics of coarse woody debris in northern coniferous forests: a synthesis. Can J for Res 34:763–777. https://doi.org/10.1139/x03-241
Leppänen SM, Salemaa M, Smolander A, Mäkipää R, Tiirola M (2013) Nitrogen fixation and methanotrophy in forest mosses along a N deposition gradient. Environ Exp Bot 90:62–69. https://doi.org/10.1016/j.envexpbot.2012.12.006
Lindo Z, Nilsson MC, Gundale MJ (2013) Bryophyte-cyanobacteria associations as regulators of the northern latitude carbon balance in response to global change. Glob Change Biol 19:2022–2035. https://doi.org/10.1111/gcb.12175
Liu X, Rousk K (2022) The moss traits that rule cyanobacterial colonization. Ann Bot 129:147–160. https://doi.org/10.1093/aob/mcab127
Mäkipää R, Leppänen SM, Munoz SS, Smolander A, Tiirola M, Tuomivirta T, Fritze H (2018) Methanotrophs are core members of the diazotroph community in decaying Norway spruce logs. Soil Biol Biochem 120:230–232. https://doi.org/10.1016/j.soilbio.2018.02.012
Markham JH (2009) Variation in moss-associated nitrogen fixation in boreal forest stands. Oecologia 161:353–359. https://doi.org/10.1007/s00442-009-1391-0
Matsumoto K, Ogawa T, Ishikawa M, Hirai A, Watanabe Y, Nakano T (2020) Organic and inorganic nitrogen deposition on the red pine forests at the northern foot of Mt. Fuji, Japan. Atmos Environ 237:117676. https://doi.org/10.1016/j.atmosenv.2020.117676
Melvin AM, Mack MC, Johnstone JF, David McGuire A, Genet H, Schuur EA (2015) Differences in ecosystem carbon distribution and nutrient cycling linked to forest tree species composition in a mid-successional boreal forest. Ecosystems 18:1472–1488. https://doi.org/10.1007/s10021-015-9912-7
Nakamura T (1984) Development of terricolous moss communities in subalpine coniferous forests of Mt. Fuji J Hattori Bot Lab 56:65–77
Nakamura T (1987) Bryophytes and lichen succession on fallen logs and seedling establishment in Tsuga-Abies forests of central Japan. Symp Biol Hung 35:485–498
Nakamura T (1992) Effect of bryophytes on survival of conifer seedlings in subalpine forests of central Japan. Ecol Res 7:155–162. https://doi.org/10.1007/BF02348494
Narukawa Y, Iida S, Tanouchi H, Abe S, Yamamoto SI (2003) State of fallen logs and the occurrence of conifer seedlings and saplings in boreal and subalpine old-growth forests in Japan. Ecol Res 18:267–277. https://doi.org/10.1046/j.1440-1703.2003.00553.x
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
Ohsawa M (1990) An interpretation of latitudinal patterns of forest limits in south and east Asian mountains. J Ecol 78:326–339. https://doi.org/10.2307/2261115
Ohsawa M (1993) Latitudinal pattern of mountain vegetation zonation in southern and eastern Asia. J Veg Sci 4:13–18. https://doi.org/10.2307/3235728
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) Vegan: community ecology package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan
Olson JB, Steppe TF, Litaker RW, Paerl HW (1998) N2-fixing microbial consortia associated with the ice cover of Lake Bonney, Antarctica. Microb Ecol 36:231–238. https://doi.org/10.1007/s002489900110
Pacé M, Paré D, Fenton NJ, Bergeron Y (2020) Effects of lichen, Sphagnum spp. and feather moss leachates on jack pine and black spruce seedling growth. Plant Soil 452:441–455. https://doi.org/10.1007/s11104-020-04587-0
Påhlsson L (1994) Vegetationstyper i Norden. Nordic Council of Ministers, Copenhagen
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Reed SC, Cleveland CC, Townsend AR (2011) Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Evol Syst 42:489–512. https://doi.org/10.1146/annurev-ecolsys-102710-145034
Renaudin M, Blasi C, Bradley RL, Bellenger JP (2022) New insights into the drivers of moss-associated nitrogen fixation and cyanobacterial biomass in the eastern Canadian boreal forest. J Ecol 110:1403–1418. https://doi.org/10.1111/1365-2745.13881
Rousk K, Jones DL, DeLuca TH (2013) Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Front Microbiol 4:150. https://doi.org/10.3389/fmicb.2013.00150
Saiz E, Sgouridis F, Drijfhout FP, Ullah S (2019) Biological nitrogen fixation in peatlands: comparison between acetylene reduction assay and 15N2 assimilation methods. Soil Biol Biochem 131:157–165
Salemaa M, Lindroos AJ, Merilä P, Mäkipää R, Smolander A (2019) N2 fixation associated with the bryophyte layer is suppressed by low levels of nitrogen deposition in boreal forests. Sci Total Environ 653:995–1004. https://doi.org/10.1016/j.scitotenv.2018.10.364
Simard MJ, Bergeron Y, Sirois L (1998) Conifer seedling recruitment in a southeastern Canadian boreal forest: the importance of substrate. J Veg Sci 9:575–582. https://doi.org/10.2307/3237273
Solheim B, Wiggen H, Røberg S, Spaink HP (2004) Associations between arctic cyanobacteria and mosses. Symbiosis 37:169–187
Sorensen PL, Lett S, Michelsen A (2012) Moss-specific changes in nitrogen fixation following two decades of warming, shading, and fertilizer addition. Plant Ecol 213:695–706. https://doi.org/10.1007/s11258-012-0034-4
Stuart JEM, Holland-Moritz H, Jean M, Miller SN, Ponciano JM, McDaniel SF, Mack MC (2021a) The relationship of C and N stable isotopes to high-latitude moss-associated N2 fixation. Oecologia 197:283–295. https://doi.org/10.1007/s00442-021-05005-7
Stuart JEM, Holland-Moritz H, Lewis LR, Jean M, Miller SN, McDaniel SF, Fierer N, Ponciano JM, Mack MC (2021b) Host identity as a driver of moss-associated N2 fixation rates in Alaska. Ecosystems 24:530–547. https://doi.org/10.1007/s10021-020-00534-3
Sugita H, Nagaike T (2005) Microsites for seedling establishment of subalpine conifers in a forest with moss-type undergrowth on Mt. Fuji, central Honshu Japan. Ecol Res 20:678–685. https://doi.org/10.1007/s11284-005-0086-1
Trifinopoulos J, Nguyen LT, Von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucl Acids Res 44:W232–W235. https://doi.org/10.1093/nar/gkw256
Wada R, Kanbayashi M, Takanashi S, Miyata T, Nakano T, Tani A, Yonemura S, Matsumi Y (2010) Investigations of effects of traffic control to nitrogen oxides on the Fuji mountain road and of atmosphere in forest site near the road. Mount Fuji Res 10:1–7 ((In Japanese))
Warshan D, Bay G, Nahar N, Wardle DA, Nilsson MC, Rasmussen U (2016) Seasonal variation in nifH abundance and expression of cyanobacterial communities associated with boreal feather mosses. ISME J 10:2198–2208. https://doi.org/10.1038/ismej.2016.17
Yamamoto S (1971) Hydrologic study of volcano Fuji and its adjacent areas. In: Tsuya H, Yamamoto S, Huzimura I, Numata M, Miyawaki A, Kuroda N (eds) Fujisan (Mt. Fuji): Report of the scientific survey of Mt. Fuji. Fuji Kyuko, Tokyo, pp 151–209
Zackrisson O, DeLuca TH, Nilsson MC, Sellstedt A, Berglund LM (2004) Nitrogen fixation increases with successional age in boreal forests. Ecology 85:3327–3334. https://doi.org/10.1890/04-0461
Zackrisson O, DeLuca TH, Gentili F, Sellstedt A, Jäderlund A (2009) Nitrogen fixation in mixed Hylocomium splendens moss communities. Oecologia 160:309–319. https://doi.org/10.1007/s00442-009-1299-8
Funding
Open access funding provided by The University of Tokyo. This work was supported by JSPS KAKENHI Grant Number JP18H02231.
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the experiments. MK performed the experiments and analyzed the data. MK wrote the manuscript; other authors provided editorial advice.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Joel Sachs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kubota, M., Matsushita, N., Nakamura, T. et al. Nitrogen fixation and nifH gene diversity in cyanobacteria living on feather mosses in a subalpine forest of Mt. Fuji. Oecologia 201, 749–760 (2023). https://doi.org/10.1007/s00442-023-05334-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-023-05334-9