Abstract
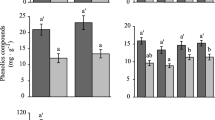
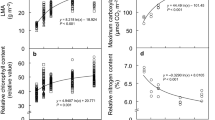
Several mistletoe species are able to grow and reproduce on both deciduous and evergreen hosts, suggesting a degree of plasticity in their ability to cope with differences in intrinsic host functions. The aim of this study was to investigate the influence of host phenology on mistletoe water relations and leaf gas exchange. Mistletoe Passovia ovata parasitizing evergreen (Miconia albicans) hosts and P. ovata parasitizing deciduous (Byrsonima verbascifolia) hosts were sampled in a Neotropical savanna. Photosynthetic parameters, diurnal cycles of stomatal conductance, pre-dawn and midday leaf water potential, and stomatal anatomical traits were measured during the peak of the dry and wet seasons, respectively. P. ovata showed distinct water-use strategies that were dependent on host phenology. For P. ovata parasitizing the deciduous host, water use efficiency (WUE; ratio of photosynthetic rate to transpirational water loss) was 2-fold lower in the dry season than in the wet season; in contrast, WUE was maintained at the same level during the wet and dry seasons in P. ovata parasitizing the evergreen host. Generally, mistletoe and host diurnal cycles of stomatal conductance were linked, although there were clear differences in leaf water potential, with mistletoe showing anisohydric behaviour and the host showing isohydric behaviour. Compared to mistletoes attached to evergreen hosts, those parasitizing deciduous hosts had a 1.4-fold lower stomatal density and 1.2-fold wider stomata on both leaf surfaces, suggesting that the latter suffered less intense drought stress. This is the first study to show morphophysiological differences in the same mistletoe species parasitizing hosts of different phenological groups. Our results provide evidence that phenotypical plasticity (anatomical and physiological) might be essential to favour the use of a greater range of hosts.




Similar content being viewed by others
References
Aasamaa K, Sõber A, Rahi M (2001) Leaf anatomical characteristics associated with shoot hydraulic conductance, stomatal conductance and stomatal sensitivity to changes of leaf water status in temperate deciduous trees. Funct Plant Biol 28:765–774
Arruda R, Fadini RF, Carvalho LN et al (2012) Ecology of Neotropical mistletoes: an important canopy-dwelling component of Brazilian ecosystems. Acta Bot Bras 26:264–274
Atsatt P (1970) Hemiparasitic flowering plants: phenotypic canalization by hosts. Nature 225:1161
Bowie M, Ward D (2004) Water and nutrient status of the mistletoe Plicosepalus acaciae parasitic on isolated Negev Desert populations of Acacia raddiana differing in level of mortality. J Arid Environ 56:487–508
Bucci SJ, Goldstein G, Meinzer FC, Scholz FG, Franco AC, Bustamante M (2004) Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant. Tree Physiol 24:891–899
Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Campanello P, Scholz FG (2005) Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in Neotropical savanna trees. Trees Struct Funct 19:296–304
Buen LL-D, Ornelas JF (1999) Frugivorous birds, host selection and the mistletoe Psittacanthus schiedeanus, in central Veracruz, Mexico. J Trop Ecol 15:329–340
Calder M, Bernhardt P (1983) The biology of mistletoes. Academic Press, Sydney
Casson S, Gray JE (2008) Influence of environmental factors on stomatal development. New Phytol 178:9–23
Clay K, Dement D, Rejmanek M (1985) Experimental evidence for host races in mistletoe (Phoradendron tomentosum). Am J Bot 72:1225–1231
Cutler J, Rains D, Loomis R (1977) The importance of cell size in the water relations of plants. Physiol Plant 40:255–260
Davidson NJ, Pate JS (1992) Water relations of the mistletoe Amyema fitzgeraldii and its host Acacia acuminata. J Exp Bot 43:1549–1555
Davidson NJ, True KC, Pate JS (1989) Water relations of the parasite: host relationship between the mistletoe Amyema linophyllum (Fenzl) Tieghem and Casuarina obesa Miq. Oecologia 80:321–330
El-Sharkawy MA, Cock JH, Hernandez ADP (1986) Differential response of stomata to air humidity in the parasitic mistletoe (Phthirusa pyrifolia) and its host, mandarin orange (Citrus resitulata). Photosynth Res 9:333–343
Escher P, Eiblmeier M, Hetzger I, Rennenberg H (2004) Spatial and seasonal variation in amino compounds in the xylem sap of a mistletoe (Viscum album) and its hosts (Populus spp. and Abies alba). Tree Physiol 24:639–650
Escher P, Peuke AD, Bannister P et al (2008) Transpiration, CO2 assimilation, WUE, and stomatal aperture in leaves of Viscum album (L.): effect of abscisic acid (ABA) in the xylem sap of its host (Populus euamericana). Plant Physiol Biochem 46:64–70
Franco AC, Bustamante M, Caldas LS et al (2005) Leaf functional traits of Neotropical savanna trees in relation to seasonal water deficit. Trees Struct Funct 19:326–335
Franklin G (1945) Preparation of thin sections of synthetic resins and wood-resin composites, and a new macerating method for wood. Nature 155:51
Galmés J, Flexas J, Savé R, Medrano H (2007) Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant Soil 290:139–155
Garkoti S, Akoijam S, Singh S (2002) Ecology of water relations between mistletoe (Taxillus vestitus) and its host oak (Quercus floribunda). Trop Ecol 43:243–249
Glatzel G, Geils BW (2009) Mistletoe ecophysiology: host–parasite interactions. Botany 87:10–15
Glazner JT, Devlin B, Ellstrand NC (1988) Biochemical and morphological evidence for host race evolution in desert mistletoe, Phoradendron californicum (Viscaceae). Plant Syst Evol 161:13–21
Herrera CM (1988) Plant size, spacing patterns, and host-plant selection in Osyris quadripartita, a hemiparasitic dioecious shrub. J Ecol 76:995–1006
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Hollinger DY (1983) Photosynthesis and water relations of the mistletoe, Phoradendron villosum, and its host, the California valley oak, Quercus lobata. Oecologia 60:396–400
Jackson PC, Meinzer FC, Bustamante M et al (1999) Partitioning of soil water among tree species in a Brazilian Cerrado ecosystem. Tree Physiol 19:717–724
Jerome CA, Ford BA (2002) The discovery of three genetic races of the dwarf mistletoe Arceuthobium americanum (Viscaceae) provides insight into the evolution of parasitic angiosperms. Mol Ecol 11:387–405
Ladley JJ, Kelly D (1996) Dispersal, germination and survival of New Zealand mistletoes (Loranthaceae): dependence on birds. N Z J Ecol 20:69–79
Lleras E (1977) Differences in stomatal number per unit area within the same species under different micro environmental conditions: a working hypothesis. Acta Amazon 7:473–476
Lüttge U, Haridasan M, Fernandes GW et al (1998) Photosynthesis of mistletoes in relation to their hosts at various sites in tropical Brazil. Trees Struct Funct 12:167–174
Maitelli G, Miranda A (1991) Evapotranspiração e fluxos de energia no Cerrado–Estação Chuvosa. An Acad Bras Cienc 63:265–272
May DS (1972) Morphological and physiological differentiation of Phoradendron populations in Texas. Am J Bot 59:12–22
Medel RG, Vergara E, Silva A, Serey IA (1995) Variation of the architectural phenotype of Tristerix aphyllus in central Chile. Rev Chilena Hist Nat 68:451–458
Meinzer F, Goldstein G, Franco AC et al (1999) Atmospheric and hydraulic limitations on transpiration in Brazilian cerrado woody species. Funct Ecol 13:273–282
Miranda A, Miranda HC, Lloyd J et al (1997) Fluxes of carbon, water and energy over Brazilian cerrado: an analysis using eddy covariance and stable isotopes. Plant Cell Environ 20:315–328
Nickrent DL, Malécot V, Vidal-Russell R, Der JP (2010) A revised classification of Santalales. Taxon 59:538–558
Okubamichael DY, Griffiths ME, Ward D (2011) Host specificity, nutrient and water dynamics of the mistletoe Viscum rotundifolium and its potential host species in the Kalahari of South Africa. J Arid Environ 75:898–902
Press MC, Graves JD (1995) Parasitic plants. Chapman & Hall Ltd, London
Press MC, Phoenix GK (2005) Impacts of parasitic plants on natural communities. New Phytol 166:737–751
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rawitscher F (1948) The water economy of the vegetation of the Campos Cerrados’ in Southern Brazil. J Ecol 36:237–268
Rawsthorne J, Watson DM, Roshier DA (2011) Implications of movement patterns of a dietary generalist for mistletoe seed dispersal. Austral Ecol 36:650–655
Rossatto DR, Hoffmann WA, Franco AC (2009) Stomatal traits of cerrado and gallery forest congeneric pairs growing in a transitional region in Central Brazil. Acta Bot Bras 23:499–508
Sachs T (2005) Pattern formation in plant tissues. Cambridge University Press, Cambridge
Scalon MC, Wright IJ (2015) A global analysis of water and nitrogen relationship between mistletoes and their hosts: broad-scale tests of old and enduring hypotheses. Funct Ecol 29:1114–1124. doi: 10.1111/1365-2435.12418
Scalon M, Haridasan M, Franco A (2013) A comparative study of aluminium and nutrient concentrations in mistletoes on aluminium-accumulating and non-accumulating hosts. Plant Biol 15:851–857
Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC (2002) Hydraulic redistribution of soil water by neotropical savanna trees. Tree Physiol 22:603–612
Shen H, Ye W, Hong L et al (2006) Progress in parasitic plant biology: host selection and nutrient transfer. Plant Biol 8:175–185
Spence R, Wu H, Sharpe P, Clark K (1986) Water stress effects on guard cell anatomy and the mechanical advantage of the epidermal cells. Plant,Cell Environ 9:197–202
Stewart GR, Press MC (1990) The physiology and biochemistry of parasitic angiosperms. Annu Rev Plant Biol 41:127–151
Ullmann I, Lange O, Ziegler H, Ehleringer J, Schulze ED, Cowan I (1985) Diurnal courses of leaf conductance and transpiration of mistletoes and their hosts in Central Australia. Oecologia 67:577–587
Vidal-Russell R, Nickrent DL (2007) The biogeographic history of Loranthaceae. Darwiniana 45:52–54
Warton DI, Duursma RA, Falster DS, Taskinen S (2012) Smatr 3—an R package for estimation and inference about allometric lines. Methods Ecol Evol 3:257–259
Xu Z, Zhou G (2008) Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot 59:3317–3325
Zuber D, Widmer A (2009) Phylogeography and host race differentiation in the European mistletoe (Viscum album L.). Mol Ecol 18:1946–1962
Acknowledgments
MCS is supported by a postdoctoral fellowship from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). ACF is recipient of a CNPq Research Productivity fellowship (303637/2011-0) and Universal-CNPq research Grant (484545/2012-4). We thank RECOR/IBGE for the logistic support and Fred Takahashi for the help in the fieldwork. This is publication number 18 in the Parasitic Plants Research Group Technical Series.
Author contribution statement
MCS, ACF and DRR conceived and designed the study. MCS, DRR and FMCBD conducted fieldwork and performed statistical analyses. MCS performed the anatomical analysis and wrote the manuscript with help from other authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hermann Heilmeier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scalon, M.C., Rossatto, D.R., Domingos, F.M.C.B. et al. Leaf morphophysiology of a Neotropical mistletoe is shaped by seasonal patterns of host leaf phenology. Oecologia 180, 1103–1112 (2016). https://doi.org/10.1007/s00442-015-3519-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3519-8