Abstract
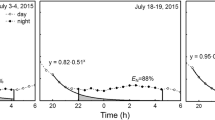
Seasonal regulation of leaf water potential (ΨL) was studied in eight dominant woody savanna species growing in Brazilian savanna (Cerrado) sites that experience a 5-month dry season. Despite marked seasonal variation in precipitation and air saturation deficit (D), seasonal differences in midday minimum ΨL were small in all of the study species. Water use and water status were regulated by a combination of plant physiological and architectural traits. Despite a nearly 3-fold increase in mean D between the wet and dry season, a sharp decline in stomatal conductance with increasing D constrained seasonal variation in minimum ΨL by limiting transpiration per unit leaf area (E). The leaf surface area per unit of sapwood area (LA/SA), a plant architectural index of potential constraints on water supply in relation to transpirational demand, was about 1.5–8 times greater in the wet season compared to the dry season for most of the species. The changes in LA/SA from the wet to the dry season resulted from a reduction in total leaf surface area per plant, which maintained or increased total leaf-specific hydraulic conductance (Gt) during the dry season. The isohydric behavior of Cerrado tree species with respect to minimum ΨL throughout the year thus was the result of strong stomatal control of evaporative losses, a decrease in total leaf surface area per tree during the dry season, an increase in total leaf-specific hydraulic conductance, and a tight coordination between gas and liquid phase conductance. In contrast with the seasonal isohydric behavior of minimum ΨL, predawn ΨL in all species was substantially lower during the dry season compared to the wet season. During the dry season, predawn ΨL was more negative than bulk soil Ψ estimated by extrapolating plots of E versus ΨL to E=0. Predawn disequilibrium between plant and soil Ψ was attributable largely to nocturnal transpiration, which ranged from 15 to 22% of the daily total. High nocturnal water loss may also have prevented internal water storage compartments from being completely refilled at night before the onset of transpiration early in the day.








Similar content being viewed by others
References
Benyon RG (1999) Night time water use in an irrigated Eucalyptus grandis plantation. Tree Physiol 19:853–864
Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Hinojosa JA, Hoffmann WA, Franco AC (2004) Processes preventing nocturnal equilibration between leaf and soil water potential in tropical savanna woody species. Tree Physiol 24:1119–1127
Clearwater MJ, Meinzer FC, Andrade JL, Goldstein G, Holbrook NM (1999) Potential errors in measurement of nonuniform sap flow using heat dissipation probes. Tree Physiol 19:681–687
Comstock JP (2000) Variation in hydraulic architecture and gas exchange in two desert sub-shrubs, Hymenoclea salsola (T. & G.) and Ambrosia dumosa. Oecologia 125:1–10
Donovan LA, Grise DJ, West JB, Pappert RA, Alder AA, Richards JH (1999) Predawn disequilibrium between plant and soil water potential in two cold desert shrubs. Oecologia 120:209–217
Donovan LA, Linton MJ, Richards JH (2001) Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia 129:328–335
Donovan LA, Richards JH, Linton MJ (2003) Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 84:463–470
Eiten G (1972) The Cerrado vegetation of central Brazil. Bot Rev 38:201–341
Ferri MG (1944) Transpiracão de plantas permanentes dos ‘cerrados’. Boletim de Facultade de Ciencias e Letras. Universidade de São Paulo. Botanica 4:1–161
Feild TS, Holbrook NM (2000) Xylem sap flow and stem hydraulics of the vesselless angiosperm Drymis granadensis (Winteraceae) in a Costa Rican elfin forest. Plant Cell Environ 23:1067–1077
Franco AC (1998) Seasonal patterns of gas exchange, water relations and growth of Roupala montana, an evergreen species. Plant Ecol 136:69–76
Goodland R, Ferri MG (1979) Ecologia do Cerrado. Editora da Universidade de São Paulo, São Paulo, Brazil
Granier A (1985) Un nouvelle méthode pour la mesure du flux de séve brut dans le tronc des arbres. Ann Sci For 42:193–200
Granier A (1987) Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiol 3:309–320
Lloyd J, Trochoulias T, Ensbey R (1991) Stomatal responses and whole-tree hydraulic conductivity or orchard Macadamia integrifolia under irrigated and non-irrigated conditions. Aus J Plant Physiol 18:661–671
Medina E, Francisco M (1994) Photosynthesis and water relations of savanna tree species differing in leaf phenology. Tree Physiol 14:1367–138
Meinzer FC, Grantz DA (1990) Stomatal and hydraulic conductance in growing sugarcane: stomatal adjustment to water transport capacity. Plant Cell Environ 13:383–388
Meinzer FC, Goldstein G, Jackson P, Holbrook NM, Gutierrez MV, Cavelier J (1995) Environmental and physiological regulation of transpiration in tropical forest gap species: the influence of boundary layer and hydraulic properties. Oecologia 101:514–522
Meinzer FC, Andrade JL, Goldstein G, Holbrook NM, Cavelier J, Jackson P (1997) Control of transpiration from the upper canopy of a tropical forest: the role of stomatal, boundary layer and hydraulic architecture components. Plant Cell Environ 20:1242–1252
Meinzer FC, Goldstein G, Franco AC, Bustamante M, Igler E, Jackson P, Caldas L, Rundel PW (1999) Atmospheric and hydraulic limitations on transpiration in Brazilian cerrado woody species. Funct Ecol 13:273–282
Miranda AC, Miranda HS, Lloyd J, Grace J, Francey RJ, Mcintyre JA, Meir P, Riggan P, Lockwood R. Brass J (1997) Fluxes of carbon, water and energy over Brazilian cerrado: an analysis using eddy covariance and stable isotopes. Plant Cell Environ 20:315–328
Oren R, Phillips N, Ewers BE, Pataki DE, Megonigal JP (1999a) Sap-flux scaled transpiration responses to light, air saturation deficit, and leaf area allocation in a flooded Taxodium distichum forest. Tree Physiol 19:337–347
Oren R, Sperry JS, Katul GG, Pataki DE, Ewers BE, Philips N, Schafer KVR (1999b) Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ 22:1515–1526
Rawitscher F (1948) The water economy of vegetation of the campos cerrados in Southern Brazil. J Ecol 36:238–268
Sarmiento G (1983) The savannas of tropical America. Elsevier, New York
Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC (2002) Hydraulic redistribution of soil water by neotropical savanna trees. Tree Physiol 22:603–612
Sperry JS, Hacke UG, Oren R, Comstock JP (2002) Water deficit and hydraulic limits to leaf water supply. Plant Cell Environ 25:251–263
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modeling isohydric and anisohydric behaviors. J Exp Bot 49:419–432
Whitehead D (1998) Regulation of stomatal conductance and transpiration in forest canopies. Tree Physiol 8:633–644
Acknowledgements
This work was supported by grants from Inter-American Institute for Global Change Research, the National Science Foundation (USA) grant 0296174, the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) and PRONEX (Brazil). We thank the Reserva Ecologica do IBGE for logistic support and meteorological data
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bucci, S.J., Goldstein, G., Meinzer, F.C. et al. Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in Neotropical savanna trees. Trees 19, 296–304 (2005). https://doi.org/10.1007/s00468-004-0391-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-004-0391-2