Abstract
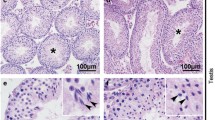
The acrosome is a specialized secretory vesicle located in the head of spermatozoa and has an essential role during fertilization. This organelle and the sperm nucleus have aberrant morphologies in forms of male infertility in humans (teratozoospermia), often associated with poor motility (asthenoteratozoospermia). To further our understanding of the aetiology of these conditions, we have performed a pathological investigation of a model of asthenoteratozoospermia that can be induced in mice by N-butyldeoxynojirimycin (NB-DNJ). We have found that, in mice treated with NB-DNJ, instead of an acrosome forming over the round spermatid nucleus, multivesicular bodies (MVB) accumulate in the vicinity of this nucleus. Electron microscopy has revealed that proacrosomic vesicles or granules (PAG) secreted during the Golgi phase of spermiogenesis do not fuse together to form an acrosomic vesicle, but rather attach transiently to the spermatid nucleus. Immunocytochemistry has shown that acrosomal membrane proteins and cytosolic acrosome-associated proteins are redirected to MVB in affected testes, whereas glycoproteins originating in the dense core of the PAG are degraded. Thus, the major effect of NB-DNJ is to inhibit membrane fusion of Golgi-derived secretory vesicles destined for acrosome formation, raising the possibility that these vesicles are critically affected in forms of (astheno)teratozoospermia.












Similar content being viewed by others
Abbreviations
- AV:
-
Acrosomic vesicle
- Gba2:
-
Beta-glucosidase
- IAM:
-
Inner acrosomal membrane
- MVB:
-
Multivesicular bodies
- NB-DNJ:
-
N-butyldeoxynojirimycin
- NE:
-
Nuclear envelope
- PAG:
-
Proacrosomic vesicles or granules
- PAS:
-
Periodic acid-Schiff
- PT:
-
Perinuclear theca
- SAL-PT:
-
Subacrosomal layer-perinuclear theca
References
Aul RB, Oko R (2002) The major subacrosomal occupant of bull spermatozoa is a novel histone H2B variant associated with the forming acrosome during spermiogenesis. Dev Biol 242:376–387
Barth AD, Oko R (1989) Normal bovine spermatogenesis and sperm maturation. Abnormal morphology of bovine spermatozoa. Iowa State University Press, Ames, pp 19–88
Bozzola JJ, Polakoski K, Haas N, Russell LD, Campbell P, Peterson RN (1991) Localization of boar sperm proacrosin during spermatogenesis and during sperm maturation in the epididymis. Am J Anat 192:129–141
Bulow M von, Heid H, Hess H, Franke WW (1995) Molecular nature of calicin, a major basic protein of the mammalian sperm head cytoskeleton. Exp Cell Res 219:407–413
Clermont Y, Tang XM (1985) Glycoprotein synthesis in the Golgi apparatus of spermatids during spermiogenesis of the rat. Anat Rec 213:33–43
Clermont Y, Oko R, Hermo L (1993) Cell and molecular biology of the testis. In: Desjardins C, Ewing L (eds) Cell biology of mammalian spermatogenesis. Oxford University Press, New York, pp 332–376
Courtade M, Lagorce C, Bujan L, Caratero C, Mieusset R (1998) Clinical characteristics and light and transmission electron microscopic sperm defects of infertile men with persistent unexplained asthenozoospermia. Fertil Steril 70:297–304
Falguieres T, Luyet PP, Gruenberg J (2009) Molecular assemblies and membrane domains in multivesicular endosome dynamics. Exp Cell Res 315:1567–1573
Hermo L, Oko R, Hecht NB (1991) Differential post-translational modifications of microtubules in cells of the seminiferous epithelium of the rat: a light and electron microscope immunocytochemical study. Anat Rec 229:31–50
Ito C, Yamatoya K, Yoshida K, Kyono K, Yao R, Noda T, Toshimori K (2010) Appearance of an oocyte activation-related substance during spermatogenesis in mice and humans. Hum Reprod 25:2734–2744
Juneja SC, Deursen JM van (2005) A mouse model of familial oligoasthenoteratozoospermia. Hum Reprod 20:881–893
Kang-Decker N, Mantchev GT, Juneja SC, McNiven MA, Deursen JM van (2001) Lack of acrosome formation in Hrb-deficient mice. Science 294:1531–1533
Kierszenbaum AL, Rivkin E, Tres LL (2003) Acroplaxome, an F-actin-keratin-containing plate, anchors the acrosome to the nucleus during shaping of the spermatid head. Mol Biol Cell 14:4628–4640
Kierszenbaum AL, Tres LL, Rivkin E, Kang-Decker N, Deursen JM van (2004) The acroplaxome is the docking site of Golgi-derived myosin Va/Rab27a/b-containing proacrosomal vesicles in wild-type and Hrb mutant mouse spermatids. Biol Reprod 70:1400–1410
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lessard JL (1988) Two monoclonal antibodies to actin: one muscle selective and one generally reactive. Cell Motil Cytoskeleton 10:349–362
Lin YN, Roy A, Yan W, Burns KH, Matzuk MM (2007) Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol Cell Biol 27:6794–6805
Machev N, Gosset P, Viville S (2005) Chromosome abnormalities in sperm from infertile men with normal somatic karyotypes: teratozoospermia. Cytogenet Genome Res 111:352–357
Meistrich ML, Trostle-Weige PK, Russell LD (1990) Abnormal manchette development in spermatids of azh/azh mutant mice. Am J Anat 188:74–86
Mountjoy JR, Xu W, McLeod D, Hyndman D, Oko R (2008) RAB2A: a major subacrosomal protein of bovine spermatozoa implicated in acrosomal biogenesis. Biol Reprod 79:223–232
Oko R (1995) Developmental expression and possible role of perinuclear theca proteins in mammalian spermatozoa. Reprod Fertil Dev 7:777–797
Oko R (1998) Occurrence and formation of cytoskeletal proteins in mammalian spermatozoa. Andrologia 30:193–206
Oko R, Clermont Y (1998) Spermiogenesis. In: Knobil E, Neill JD (eds) Encyclopedia of reproduction. Academic Press, San Diego, pp 602–609
Oko R, Maravei D (1994) Protein composition of the perinuclear theca of bull spermatozoa. Biol Reprod 50:1000–1014
Oko R, Maravei D (1995) Distribution and possible role of perinuclear theca proteins during bovine spermiogenesis. Microsc Res Tech 32:520–532
Oko R, Sutovsky P (2009) Biogenesis of sperm perinuclear theca and its role in sperm functional competence and fertilization. J Reprod Immunol 83:2–7
Oko R, Hermo L, Hecht NB (1991) Distribution of actin isoforms within cells of the seminiferous epithelium of the rat testis: evidence for a muscle form of actin in spermatids. Anat Rec 231:63–81
Oko RJ, Jando V, Wagner CL, Kistler WS, Hermo LS (1996) Chromatin reorganization in rat spermatids during the disappearance of testis-specific histone, H1t, and the appearance of transition proteins TP1 and TP2. Biol Reprod 54:1141–1157
Raiborg C, Rusten TE, Stenmark H (2003) Protein sorting into multivesicular endosomes. Curr Opin Cell Biol 15:446–455
Russell LD, Russell JA, MacGregor GR, Meistrich ML (1991) Linkage of manchette microtubules to the nuclear envelope and observations of the role of the manchette in nuclear shaping during spermiogenesis in rodents. Am J Anat 192:97–120
Smith CE, Hermo L, Fazel A, Lalli MF, Bergeron JJ (1990) Ultrastructural distribution of NADPase within the Golgi apparatus and lysosomes of mammalian cells. Prog Histochem Cytochem 21:1–120
Sotomayor RE, Handel MA (1986) Failure of acrosome assembly in a male sterile mouse mutant. Biol Reprod 34:171–182
Spoel AC van der, Jeyakumar M, Butters TD, Charlton HM, Moore HD, Dwek RA, Platt FM (2002) Reversible infertility in male mice after oral administration of alkylated imino sugars: a nonhormonal approach to male contraception. Proc Natl Acad Sci USA 99:17173–17178
Stahl PD, Barbieri MA (2002) Multivesicular bodies and multivesicular endosomes: the "ins and outs" of endosomal traffic. Sci STKE 2002:E32
Suganuma R, Walden CM, Butters TD, Platt FM, Dwek RA, Yanagimachi R, Spoel AC van der (2005) Alkylated imino sugars, reversible male infertility-inducing agents, do not affect the genetic integrity of male mouse germ cells during short-term treatment despite induction of sperm deformities. Biol Reprod 72:805–813
Susi FR, Leblond CP, Clermont Y (1971) Changes in the Golgi apparatus during spermiogenesis in the rat. Am J Anat 130:251–267
Szczygiel M, Kurpisz M (1999) Teratozoospermia and its effect on male fertility potential. Andrologia 31:63–75
Tang XM, Lalli MF, Clermont Y (1982) A cytochemical study of the Golgi apparatus of the spermatid during spermiogenesis in the rat. Am J Anat 163:283–294
Thorne-Tjomsland G, Clermont Y, Hermo L (1988) Contribution of the Golgi apparatus components to the formation of the acrosomic system and chromatoid body in rat spermatids. Anat Rec 221:591–598
Tovich PR, Sutovsky P, Oko RJ (2004) Novel aspect of perinuclear theca assembly revealed by immunolocalization of non-nuclear somatic histones during bovine spermiogenesis. Biol Reprod 71:1182–1194
Walden CM, Sandhoff R, Chuang CC, Yildiz Y, Butters TD, Dwek RA, Platt FM, Spoel AC van der (2007) Accumulation of glucosylceramide in murine testis, caused by inhibition of beta-glucosidase 2: implications for spermatogenesis. J Biol Chem 282:32655–32664
Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, Day BN, Park KW, Yi YJ, Xi YW, Prather RS, Oko R (2007a) PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem 282:12164–12175
Wu AT, Sutovsky P, Xu W, Spoel AC van der, Platt FM, Oko R (2007b) The postacrosomal assembly of sperm head protein, PAWP, is independent of acrosome formation and dependent on microtubular manchette transport. Dev Biol 312:471–483
Yildiz Y, Matern H, Thompson B, Allegood JC, Warren RL, Ramirez DM, Hammer RE, Hamra FK, Matern S, Russell DW (2006) Mutation of beta-glucosidase 2 causes glycolipid storage disease and impaired male fertility. J Clin Invest 116:2985–2994
Yu Y, Xu W, Yi YJ, Sutovsky P, Oko R (2006) The extracellular protein coat of the inner acrosomal membrane is involved in zona pellucida binding and penetration during fertilization: characterization of its most prominent polypeptide (IAM38). Dev Biol 290:32–43
Yu Y, Vanhorne J, Oko R (2009) The origin and assembly of a zona pellucida binding protein, IAM38, during spermiogenesis. Microsc Res Tech 72:558–565
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants to R.O. from CIHR (MOP-84440) and NSERC (RGPIN/192093) of Canada and to A.C.S. from NIH/NICHD (U01 HD45861).
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Fig. S1
Effect of NB-DNJ on the expression of actin. Sections of normal (a) and NB-DNJ-affected (b, c) testes at stages VII and VIII after being immunoperoxidase-stained with anti-C4 monoclonal antibody (Lessard 1988), which generally recognizes all non-muscle and muscle vertebrae actins, and counterstained with Periodic acid-Schiff (PAS) and methylene blue. a Actin immunostaining first becomes prominent in the acrosomal region of control step-8 spermatids (8). In contrast, little to no actin immunostaining is seen over PAS-stained acrosomes in control step-7 spermatids (7) in the same section, even though actin immunoreactivity is present throughout the cytoplasm of round spermatids. b, c In contrast, immunoreactivity and PAS staining are not detectable in the acrosomal region in comparable steps of spermiogenesis in NB-DNJ-treated mice. Bars 5 μm. (JPEG 181 kb)
Fig. S2
Effect of NB-DNJ on the expression of integral membrane protein, IAM32. Sections of normal (a) and NB-DNJ-treated (b) testes at stage VII after being immunoperoxidase-stained with anti-IAM32 antibodies and counterstained with methylene blue. The immunostaining of the acrosomal cap (arrows) in control round spermatids is replaced by immunostaining of Multivesicular bodies (MVB, arrows) in the cytoplasm of NB-DNJ-affected spermatids. These MVB are also apparent in the cytoplasm of elongated spermatids. Bars 5 μm. (JPEG 85 kb)
Rights and permissions
About this article
Cite this article
Oko, R., Donald, A., Xu, W. et al. Fusion failure of dense-cored proacrosomal vesicles in an inducible mouse model of male infertility. Cell Tissue Res 346, 119–134 (2011). https://doi.org/10.1007/s00441-011-1248-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1248-9