Abstract
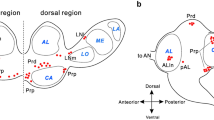
CYCLE (CYC) and CLOCK (CLK) are transcriptional activators of the circadian clock genes, period (per) and timeless (tim), binding at E-boxes of their upstream regulatory region in Drosophila. CYC-like and CLK-like immunohistochemical reactivities (CYC-ir and CLK-ir) were investigated in the ground cricket, Allonemobius allardi, in which immunohistochemical reactivities for three circadian clock proteins (PERIOD, Doubletime, and Cryptochrome), two neuropeptides (crustacean cardioactive peptide and diapause hormone), and arylalkylamine-N-acetyltransferase had previously been mapped in the brain-subesophageal ganglion (SOG) complex. CYC-ir and CLK-ir occurred predominantly in the cytoplasm of the neurons distributed mainly in the central brain, SOG, and corpora cardiaca. Double-labeling experiments showed that CYC-ir and CLK-ir were co-localized only in the mandibular and maxillary neuromeres of the SOG. The neuronal processes in the dorsolateral region of the protocerebrum partially shared the immunoreactivities, whereas most of the other immunoreactivities were unique. The optic lobe showed reactivity to anti-CYC at small proximal frontodorsal cells and to anti-CLK at small proximal frontoventral cells. The frontal ganglion exhibited CYC-ir in the cell bodies that lacked CLK-ir. No difference in their number, distribution, or staining intensity was found between sampling under light:dark regimes of 16:8 and 12:12. The levels of both CYC-ir and CLK-ir showed no oscillation throughout a 24-h period. The co-localization pattern suggests that the midline cells of the SOG share most of the circadian-related immunoreactivities, thus constituting the heart of the circadian clock in A. allardi.





Similar content being viewed by others
References
Abe Y, Ushirogawa H, Tomioka K (1997) Circadian locomotor rhythm of the cricket, Gyllodes sigillatus. I. Localization of the pacemaker and the photoreceptor. Zool Sci 14:719–727
Allada R, White NE, So WV, Hall JC, Rosbash M (1998) A mutant Drosophila homology of mammalian clock disrupts circadian rhythms and transcription of period and timeless. Cell 93:791–804
Bae K, Lee C, Hardin PE, Edery I (2000) dCLOCK is present in limiting amounts and likely mediates daily interactions between the dCLOCK-CYC transcription factor and the PER-TIM complex. J Neurosci 20:1746–1753
Blau J, Young MW (1999) Cycling Vrille expression is required for a functional Drosophila clock. Cell 99:661–671
Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA (2002) Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci 22:9305–9319
Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW (2001) Circadian regulation of gene expression systems in the Drosophila head. Neuron 32:657–671
Cyran SA, Buchsbaum AM, Reddy KL, Lin MC, Glossop NR, Hardin PE, Young MW, Storti RV, Blau J (2003) vrille, Pdp1 and dClock form a second feed back loop in the Drosophila circadian clock. Cell 112:329–341
Darlington TK, Wagner-Smith K, Ceriani MF, Staknis D, Gekasis N, Steeves TDL, Weitz CJ, Takahashi JS, Key SA (1998) Closing the circadian loop: clock-induce transcription of its own inhibitors per and tim. Science 280:1599–1603
Ewer J, Frisch B, Hamblen-Coyle MJ, Rosbash M, Hall JC (1992) Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells influence on circadian behavioral rhythms. J Neurosci 12:3321–3349
Frisch B, Hardin PE, Hamblen-Coyle MJ, Rosbash M, Hall JC (1994) A promoterless period gene mediates behavioral rhythmicity and cyclical per expression in a restricted subset of the Drosophila nervous system. Neuron 12:555–570
Frisch B, Fleissner G, Brandes C, Hall JC (1996) Staining in the brain of Pachymorpha sexguttata mediated by an antibody against a Drosophila clock-gene product: labeling of cells with possible importance for the beetle’s circadian rhythms. Cell Tissue Res 286:411–429
Glossop NR, Houl JH, Zheng H, Ng FS, Dudek SM, Hardin PE (2003) VRILLE feeds back to control circadian transcription of clock in the Drosophila circadian oscillator. Neuron 37:249–261
Hardin PE (2004) Transcription regulation within the circadian clock: the E-box and beyond. J Biol Rhythms 19:348–360
Hardin PE, Hall JC, Rosbash M (1992) Behavioral and molecular analysis suggests that circadian output is disrupted by disconnected mutants in D. melanogaster. EMBO J 11:1–6
Helfrich-Förster C (1998) Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol [A] 182:435–453
Helfrich-Förster C (2004) The circadian clock in the brain: a structural and functional comparison between mammals and insects. J Comp Physiol [A] 190:601–613
Helfrich-Förster C (2005) Organization of endogenous clocks in insects. Biochem Soc Trans 33:957–961
Houl JH, Yu W, Dudek SM, Hardin PE (2006) Drosophila CLOCK is constitutively expressed in circadian oscillator and non-oscillator cells. J Biol Rhythms 21:93–103
Lee C, Bae K, Edery I (1998) The Drosophila CLOCK protein undergoes daily rhythms in abundance, phosphorylation, and interactions with PER-TIM complex. Neuron 21:857–867
Markova EP, Shimada T, Takeda M (2003b) Daily expression patterns of cycle and clock genes in the head of the silkworm, Bombyx mori. J Biotech Biotechnol Equip 18:77–81
McDonald MJ, Rosbash M (2001) Microarray analysis and organization of circadian gene expression in Drosophila. Cell 107:567–578
Nishiitsutsuji-Uwo J, Pittendrigh CS (1968) Central nervous system control of circadian rhythmicity in the cockroach. II. The pathway of light signals that entrain the rhythm. Z Vergl Physiol 58:1–13
Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99:791–802
Rutila JE, Suri V, Le M, So WV, Rosbash M, Hall JC (1998) CYCLE is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 93:805–814
Sauman I, Reppert SM (1996) Circadian clock neurons in the silkmoth Antheraea pernyi: novel mechanisms of Period protein regulation.Neuron 17:889–900
Schneider NL, Stengl M (2005) Pigment-dispersing factor and GABA synchronize cells of the isolated circadian clock of the cockroach Leucophaea maderae. J Neurosci 25:5138–5147
Sehadová H, Markova EP, Sehnal F, Takeda M (2004) Distribution of circadian clock-related proteins in the cephalic nervous system of the silkworm, Bombyx mori. J Biol Rhythms 19:466–482
Sehadová H, Shao QM, Sehnal F, Takeda M (2007) Neurohormones as putative circadian clock output signals in the central nervous system of two cricket species. Cell Tissue Res 328:239–255
Shao QM, Sehadová H, Ichihara N, Sehnal F, Takeda M (2006) Immunoreactivities to three circadian clock proteins in two ground crickets suggest interspecific diversity of the circadian clock structure. J Biol Rhythms 21:118–131
Shimizu T, Masaki S (1997) Geographical and species variation in circadian rhythm parameters in nemobiine crickets. Physiol Entomol 22:83–93
Sokolove PG (1975) Localization of the cockroach optic lobe circadian pacemaker with microlesions. Brain Res 87:13–21
Tomioka K, Chiba Y (1984) Effects of nymphal stage optic nerve severance of optic lobe removal on the circadian rhythm of the cricket, Gryllus bimaculatus. Zool Sci 1:385–394
Tomioka K, Abdelsalam S (2004) Circadian organization in hemimetabolous insects. Zool Sci 21:1153–1162
Ueda HR, Matsumoto A, Kawamura M, Iino M, Tanimura T, Hashimoto S (2002) Genome-wide transcriptional orchestration of circadian rhythm in Drosophila. J Biol Chem 277:14048–14052
Wang GK, Ousley A, Darlington TK, Chen D, Chen Y, Fu W, Hickman LJ, Kay SA, Sehgal A (2001) Regulation of the cycling of timeless (tim) RNA. J Neurobiol 47:161–175
Wise S, Davis NT, Tyndale E, Noveral J, Folwell MG, Bedian V, Emery IF, Siwicki KK (2002) Neuroanatomical studies of period gene expression in the hawkmoth, Manduca sexta. J Comp Neurol 447:366–380
Závodská R, Sauman I, Sehnal F (2003a) Distribution of PER protein, pigment-dispersing hormone, prothoracicotropic hormone, and eclosion hormone in the cephalic nervous system of insects. J Biol Rhythms 18:106–122
Závodská R, Sauman I, Sehnal F (2003b) The cycling and distribution of PER-like antigen in relation to neurons recognized by the antisera to PTTH and EH in Thermobia domestica. Insect Biochem Mol Biol 33:1227–1238
Zhu H, Yuan Q, Briscoe AD, Froy O, Casselman A, Reppert SM (2005) The two CRYs of butterfly. Curr Biol 15:R953–R954
Acknowledgments
We thank Dr. M. Takagi who helped us on production of antibodies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shao, QM., Hiragaki, S. & Takeda, M. Co-localization and unique distributions of two clock proteins CYCLE and CLOCK in the cephalic ganglia of the ground cricket, Allonemobius allardi . Cell Tissue Res 331, 435–446 (2008). https://doi.org/10.1007/s00441-007-0534-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0534-z