Abstract
Purpose
We used a within-subject, cross-over study to determine the relationship between the intra-individual adaptations to four weeks’ resistance (RT) versus four weeks’ endurance (END) training, and we investigated whether three single nucleotide polymorphisms (SNPs) were associated with these adaptations.
Methods
Thirty untrained, healthy, young men completed a cycling test to exhaustion to determine peak oxygen uptake (V̇O2peak), and a knee extension (KE) maximum voluntary isometric contraction (MVIC) of the right leg before and after four weeks’ supervised RT (four sets of 10 repetitions at 80% single repetition maximum unilateral KE exercise, three times weekly) and four weeks’ supervised END (30 min combined continuous/interval cycling, three times weekly), separated by a three-week washout phase. Participants were genotyped for the ACTN3 rs1815739, NOS3 rs2070744 and VEGFA rs2010963 SNPs.
Results
The intra-individual adaptations regarding percentage changes in MVIC force and V̇O2peak following RT and END, respectively, were unrelated (r2 = 0.003; P = 0.79). However, a VEGFA genotype × training modality interaction (P = 0.007) demonstrated that VEGFA GG homozygotes increased their MVIC force after RT (+ 20.9 ± 13.2%) more than they increased their V̇O2peak after END (+ 8.4 ± 9.1%, P = 0.005), and more than VEGFA C-allele carriers increased their MVIC force after RT (+ 12.2 ± 8.1%, P = 0.04). There were no genotype × training modality interactions for the ACTN3 or NOS3 SNPs.
Conclusion
High/low responders to RT were not consequently high/low responders to END or vice versa. However, preferential adaptation of VEGFA rs2010963 GG homozygotes to RT over END, and their greater adaptation to RT compared to VEGFA C-allele carriers, indicate a novel genetic predisposition for superior RT adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Short-term (e.g. three to five weeks) resistance training (RT) is a potent stimulus for increasing muscle strength (Moritani and deVries 1979; Seynnes et al. 2007; Buckthorpe et al. 2015), while short-term endurance training (END) increases peak oxygen uptake (V̇O2peak) (Dunham and Harms 2012; Murias et al. 2016; Dawson et al. 2021). However, until relatively recently, studies focussed on the mean adaptation to these chronic exercise modalities, while variation around the mean was primarily attributed to measurement error. It has since become apparent that the variable response to RT (Hubal et al. 2005; Erskine et al. 2010a) and END (Bouchard et al. 1999) cannot be simply explained by measurement error alone, and understanding this variation may in fact elucidate novel mechanisms controlling gains in muscular strength, as well as cardiovascular fitness. However, it is not yet known if the variable adaptations to dichotomous modalities of short-term exercise training, i.e. RT and END, are related within individuals. In other words, are high responders to short-term RT (in terms of strength gains) likely to show a poor response to END (in terms of V̇O2peak) or vice versa. Alternatively, will high responders to one mode of chronic exercise also respond positively to the other mode of exercise? A recent cross-over training study found no relationship between strength gains following 12 weeks’ RT and changes in V̇O2peak following 12 weeks’ END (Marsh et al. 2020). However, this study incorporated multiple types of RT (e.g. upper- and lower-limb) and END (e.g. running and cycling) of varying intensities and volume. Such an approach may introduce overlap in the effects of both exercise modalities, and therefore make it difficult to directly compare the effects of RT with END in the same group of individuals. More specific training modalities and assessments would improve our understanding of the physiological mechanisms underpinning the adaptations to dichotomous modes of short-term chronic exercise, while providing evidence to support or refute the case for personalised exercise prescription to optimise the health benefits of exercise.
Furthermore, the variable response to both RT (Thomis et al. 1998) and END (Bouchard et al. 1999) in previously untrained individuals is known to have significant heritability, i.e. the genetic component is suggested to explain ~ 20% of the change in strength and ~ 47% of the change in V̇O2peak. Moreover, there is evidence that variation within specific genes (e.g. single nucleotide polymorphisms, SNPs) influence the adaptation to chronic exercise, although this evidence is based on single modality exercise programmes. For example, the α-actinin-3 (ACTN3) gene encodes the α-actinin-3 protein that anchors actin to the Z-disk in human type II skeletal muscle fibres and plays an instrumental role in hindering the conversion of the larger, stronger, faster type II fibre characteristics to smaller, weaker, more oxidative type I fibre properties (Seto et al. 2013). Some studies (Delmonico et al. 2007), but not all (Clarkson et al. 2005; Erskine et al. 2014), suggest that ACTN3 rs1815739 RR homozygotes demonstrate greater improvements in maximal strength and power following chronic RT, while there is evidence that ACTN3 rs1815739 XX homozygotes demonstrate greater improvements in V̇O2peak following END [see review of Del Coso et al. (2019)]. However, no study has yet investigated if this SNP may help to explain potential intra-individual variability in the adaptations to RT and END.
Another gene of interest regarding the variable response to chronic exercise is the nitric oxide (NO) synthase 3 (NOS3) gene, which encodes endothelial NO synthase (eNOS) (Marsden et al. 1993), and is related to vascular health (Zmijewski et al. 2018). For example, systolic and diastolic blood pressure have been shown to decrease after 12 weeks’ END only in NOS3 rs2070744 TT homozygotes and not C-allele carriers (Trapé et al. 2021), suggesting that NOS3 TT genotype influences a positive vascular adaptation to END. Furthermore, we have recently shown that NOS3 rs2070744 TT homozygotes improved their vascular function after END, but not after RT (Dawson et al. 2021). NOS3 C-allele carriers, on the other hand, showed a tendency to improve vascular function following RT but not END (Dawson et al. 2021), thus providing evidence that a genetic association with the adaptation to chronic exercise training is specific to the mode of exercise. The T-allele has also been linked to elite power athlete status and superior sprint/power performance (Murtagh et al. 2020), suggesting it may be associated with superior adaptations to RT over END within the same group of individuals, although this has yet to be investigated.
A third gene that may influence the intra-individual adaptations to RT and END in humans is the vascular endothelial growth factor-A (VEGFA) gene, which encodes the VEGF-A protein that regulates erythropoiesis, angiogenesis, and muscle blood flow (Dai and Rabie 2007). Specific SNPs (e.g. rs2010963) within the VEGFA gene have been associated with endurance performance in humans (Prior et al. 2006) and with an increase in capillary density following END in mice (Booth et al. 2015) but not RT in humans (Campos et al. 2002). However, no study has investigated whether variations of this gene are associated with the intra-individual adaptations to RT and END in humans. In fact, it is not yet known if variation in any genes can help explain differences in the adaptations (with respect to strength gains and improvements in cardiovascular fitness) to RT and END within the same group of individuals.
We therefore used a within-subject, cross-over design to determine the relationship between the adaptations to four weeks’ RT (in terms of strength gains) versus four weeks’ END (in terms of changes in V̇O2peak) within the same group of previously untrained, healthy, young men. Further, we investigated whether SNPs within the ACTN3, NOS3, and VEGFA genes were associated with intra-individual differences in the adaptations to RT versus END. We hypothesised that individuals carrying at least one ACTN3 rs1815739 R-allele (RR + RX genotypes), and those homozygous for the NOS3 rs2070744 T-allele (TT genotypes) would demonstrate greater RT than END adaptations, and that these adaptations would be the opposite to those demonstrated by their ACTN3 XX homozygote and NOS3 C-allele carrying counterparts. We also hypothesised that carriers of the VEGFA rs2010963 C-allele would adapt better to END than RT, while GG homozygotes would demonstrate superior adaptations to RT than END.
Methods
Study design and participant recruitment
Forty previously untrained (but recreationally active), healthy, young men from the student population at Liverpool John Moores University volunteered to take part in this study. A minimal sample size was estimated a priori with G*Power software (v3.1.9.6, Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany). The estimation was performed using a small-to-medium effect size (ƞp2 = 0.05; α = 0.05; power = 0.80), based on the RT-induced peak power gains in male ACTN3 rs1815739 RR vs. XX homozygotes in the study by (Delmonico et al. 2007). A minimum of 28 participants was deemed necessary to detect a genotype × exercise modality interaction effect. However, due to the relatively demanding nature of the study design (e.g. frequency of training sessions and overall study duration), we expected ~ 20% participants would drop out, so we increased our initial sample size to n = 40. Indeed, as anticipated, 10 participants withdrew due to personal reasons at various stages of the study, and 30 participants (height: 1.79 ± 0.07 m; body mass: 77.0 ± 9.7 kg; age: 21 ± 2 years) completed the study. Volunteers with history of lower-limb musculoskeletal injuries or cardiovascular disease, or who reported cardiovascular risk factors or were using any medication or nutritional supplementation that could potentially affect physical performance, or who smoked, were excluded from the study. All participants gave written informed consent before taking part in the study, which was approved by Liverpool John Moores University Research Ethics Committee (approval number: 13/APS/032) and adhered to the Declaration of Helsinki.
Experimental design
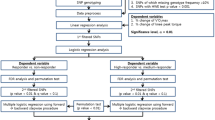
Before and after both four-week exercise training programmes, all participants reported to the laboratory on two occasions to undergo testing procedures, separated by at least 24 h between visits. During the first visit, all underwent anthropometric measurements and a cycling test to exhaustion with gas exchange analysis to determine V̇O2peak, and all provided a venous blood sample, from which DNA was isolated for genotyping. During the second visit, knee extenstion (KE) maximum voluntary isometric contraction (MVIC) force of the right leg was measured. This order was kept the same throughout the entire protocol. Participants completed 12 sessions over a four-week period, either RT or END training in a randomised, balanced cross-over design (Fig. 1). Detraining adaptations (following either END or RT) seem to follow a similar time course to that of training adaptations in young, untrained individuals, i.e. three to four weeks’ END or RT elicit a 10–15% increase in either V̇O2peak or MVIC force, while similar reductions in these variables are observed after three to four weeks’ detraining (Narici et al. 1989; Neufer 1989). Thus, in between each four-week END or RT training block, our participants completed a washout period of three weeks to ensure V̇O2peak or MVIC force had returned to baseline prior to the next four-week training period (Fig. 2). For each participant, all testing at baseline, after the first four weeks’ training block, after the three-week washout period, and after the second four weeks’ training block were completed within a seven-day period of the first/last training session.
Measurements
Cardiopulmonary exercise testing (CPET) was performed in all participants on an electronically braked cycle ergometer (Daum-electronic premium, 8i ergo-bike, Fürth, Germany). V̇O2peak (mL∙kg−1∙min−1), and respiratory exchange ratio (RER) were measured continuously at rest and during exercise using a metabolic system (Metamax 3B, MM3B, Cortex, Leipzig, Germany). Measurements also included power output (Watts, W), heart rate (HR, beats per minute, bpm) with a Polar FT1 heart rate monitor with a Pro chest strap Strap (Polar Electro Oy, Kempele, Finland). The incremental protocol began with a power output of 95 W, followed by an increase of 35 W every 3 min until exhaustion, while maintaining a cadence of 80 rpm. The oxygen uptake (V̇O2) was considered to have peaked (V̇O2peak) when at least two of the three following criteria were met: (i) a levelling off of V̇O2 with increasing intensity (an increase of no more than 2 ml∙kg−1∙min−1); (ii) a HR within 10 beats∙min−1 of the age-predicted maximum (220 minus age in years); (iii) a respiratory exchange ratio (RER) greater than 1.05. V̇O2peak was determined from the mean of the three consecutive highest values over a 30-s interval reached during the last stage of the protocol. Strong verbal encouragement was given throughout the test. This was followed by 15 min’ unloaded recovery cycling at a self-selected cadence.
Knee extension MVIC force
Participants were secured with inextensible straps to in an isokinetic dynamometer (IKD) chair (Lido Active, Loredan, Davis, CA, USA), with a hip angle of 90° (180° = supine) and a 90° knee flexion angle (0° = full knee extension). The right ankle was connected to the force transducer (KAP, Bienfait B.V. Haarlem, The Netherlands) and the force signal was interfaced with an analog-to-digital converter (MP150, Biopac Systems, CA, USA), sampled at 2000 Hz with a PC using Acqknowledge software (version 5, Biopac) and low-pass filtered (10-Hz). After a warm-up of 10 submaximal voluntary contractions, with participants increasing their force output each time, participants were instructed to perform two MVICs, separated by a one-minute rest period. If the second MVIC was > 5% higher than the first, a third attempt was performed until the highest MVIC was < 5% higher than the next highest. Verbal encouragement was provided by researchers and biofeedback was provided to participants by projecting their MVIC attempts on a screen in front of them in real time.
DNA extraction and genotyping
A blood sample was drawn into a 10-mL EDTA vacutainer (BD Vacutainer Systems, Plymouth, UK) from a superficial forearm vein. The whole blood was aliquoted into 2-mL tubes (Eppendorf AG, Hamburg, Germany) and stored at −80 °C until subsequent analysis. DNA purification from whole blood samples was performed manually using a QIAamp DNA Blood Mini Kit (Qiagen Ltd., Manchester, UK), following the manufacturer’s guidelines, as described previously (Erskine et al. 2012). Samples of DNA were then stored at 4 °C until subsequent genotyping. Real-time polymerase chain reaction was performed (Rotor-Gene Q, Qiagen) to establish the genotypes of the ACTN3 rs1815739, NOS3 rs2070744, and VEGFA rs2010963 SNPs for each participant. Each 10 μL reaction volume contained 5 μL Genotyping Master Mix (Applied Biosystems, Foster City, USA), 3.5 μL nuclease-free H2O (Qiagen), 0.5 μL SNP (ACTN3, NOS3 or VEGFA) TaqMan genotyping assay (Applied Biosystems), plus 1 μL DNA sample. Both negative [1 μL nuclease-free H2O (Qiagen) replaced the DNA template] and positive controls were included in each RT-PCR run, which used the following protocol: denaturation at 95 °C for 10 min, followed by 40 to 50 cycles of incubation at 92 °C for 15 s, then annealing and extension at 60 °C for 1 min. Genotypes were determined using Rotor-Gene Q Pure Detection 2.1.0 software (Qiagen). All samples were analysed in duplicate and there was 100% agreement between genotypes calls for samples from the same participant.
Exercise training
Resistance training
All participants completed all 12 training sessions, which were supervised by members of the research team and were performed three times a week for four weeks. The RT was performed on a leg extension machine (Technogym, Gambettola, Italy) by alternating one leg at a time. Prior to the first training session of each week, the leg extension single repetition maximum (1-RM) was assessed to determine the training load for that week and ensure the load was progressively increased (each week, the training load was adjusted according to the new 1-RM) (Haff et al. 2015). Before each RT session, a warm-up set of 10 repetitions at 40% 1-RM was performed, which was followed by 4 sets of 10 repetitions at 80% 1-RM for each leg, with 2 min recovery between sets.
Endurance training
All participants completed all 12 training sessions, which were supervised by members of the research team and were performed three times a week for four weeks. Each 30 min END session comprised cycling on a cycle ergometer (Lode BV, Groningen, the Netherlands), with intensity progressively increased on a weekly basis. Week 1 comprised steady-state (SS) cycling at 70% maximal HR (HRmax, assessed during the CPET); week 2 comprised SS at 70% HRmax interspersed every sixth min with 1 min at 90% HRmax; week 3 comprised SS at 80% HRmax; week 4 comprised SS at 80% HRmax interspersed every sixth min with 1 min at 90% HRmax. Before and after each training session, participants performed a 3-min warm-up/cool-down at 60–80 W.
Statistical analysis
Data are presented as mean ± standard deviation. The statistical analyses were performed with GraphPad Prism 9.0.1 (GraphPad Software, Inc., La Jolla, California, USA). Differences were defined as statistically significant when P < 0.05. After ensuring a normal distribution, bivariate Pearson’s correlations were used to determine relationships between intra-individual percentage changes in MVIC force after RT and percentage changes in V̇O2peak after END. A one-way analysis of variance (ANOVA) was used to compare baseline values between the two exercise modalities. A two-way ANOVA with repeated measures (exercise modality × time) was used to compare body mass, blood pressure, and heart rate between the two training interventions. Genotype frequency distributions for all three SNPs were tested for compliance with Hardy–Weinberg Equilibrium using Pearson’s χ2 tests. A two-way mixed ANOVA was used to compare percentage change in MVIC force after RT with percentage change in V̇O2peak after END between genotypes for each SNP: ACTN3 (RR + RX vs. XX), NOS3 (CC + TC vs. TT), and VEGFA (CC + GC vs. GG). In the case of a significant two-way interaction, post hoc paired t tests were used to determine the preferential exercise adaptation within-genotype, while post hoc independent t tests were used to determine the preferential exercise adaptation between genotypes. Effect sizes for the two-way mixed ANOVA interactions (partial eta-squared, ηp2), and t tests (Cohen’s d) were reported for each statistical model. The thresholds of Cohen’s d and ηp2 are defined as small (d = 0.20 and ηp2 = 0.01), medium (d = 0.50 and ηp2 = 0.06) and large (d = 0.80 and ηp2 = 0.14) (Cohen 1988; Bakeman 2005). A false discovery rate (FDR) of 10% was used to control for multiple comparisons (Benjamini and Hochberg 1995).
Results
When categorised according the genotype groups for each SNP, none of the baseline characteristics (Table 1) differed between groups, except for age between NOS3 CC + TC and TT genotypes (P = 0.02). Body mass, blood pressure and resting heart rate did not change after either RT or END. No order effect for training modality was found for either MVIC force or V̇O2peak (P > 0.05). The genotype and allele frequency distributions for the three SNPs were as follows:
ACTN3 rs1815739 genotype: RR, n = 9 (0.30); RX, n = 13 (0.43); XX, n = 8 (0.27); allele: R, 0.52; X, 0.48;
NOS3 rs2070744 genotype: TT, n = 12 (0.40); CT, n = 14 (0.47); CC, n = 4 (0.13); allele: T, 0.63; C, 0.37;
VEGFA rs2010963 genotype: GG n = 15 (0.50), GC n = 13 (0.43), CC n = 2 (0.07); allele: G, 0.72; C, 0.28.
Genotype frequency distributions for each SNP were in Hardy–Weinberg Equilibrium (χ2 ≤ 0.535; P ≥ 0.765).
Between-exercise modality comparison
We found a time × exercise modality interaction for knee extensor MVIC and V̇O2peak (P < 0.001 for both) with a greater improvement of knee extensor MVIC force after RT and a greater improvement of V̇O2peak after END after Bonferroni post hoc analyses (P < 0.001). Post hoc paired t tests demonstrated that RT improved MVIC force (P < 0.001), but not V̇O2peak after the four-week training intervention (Fig. 2A). Similarly, END improved V̇O2peak (P < 0.001), but not MVIC force (Fig. 2B). Paired t tests confirmed that V̇O2peak (pre-END vs. pre-RT, P = 0.20) and MVIC force (pre-RT vs. pre-END, P = 0.76) had returned to baseline values after the three-week washout period.
Within-individual comparison
When comparing the individual percentage change in knee extensor MVIC force (Fig. 3) after RT versus respective changes in V̇O2peak after END in all 30 participants, we observed large intra- and inter-individual variation. However, the individual changes in MVIC force after RT did not correlate with the changes in V̇O2peak after END (r2 = 0.003; P = 0.79).
ACTN3 rs1815739 genotype
No significant genotype × exercise modality interaction was found for the change in MVIC force and V̇O2peak following RT and END, respectively (P = 0.93, ηp2 < 0.001, Fig. 4A). We found a tendency for a main effect of ACTN3 genotype regarding a training-induced change in MVIC force and V̇O2peak, with XX homozygotes tending to display larger changes in MVIC force and V̇O2peak compared to R-allele carriers (RR + RX genotypes) (P = 0.07, Fig. 4A).
Percentage changes in knee extensor maximum voluntary isometric contraction (MVIC) force after resistance training (RT) compared to change in peak oxygen uptake (V̇O2peak) after endurance training (END) in individuals of ACTN3 rs1815739 RR + RX and XX (A), NOS3 rs2070744 CC + TC and TT (B), and VEGFA rs2010963 CC + GC and GG (C) genotypes; *within-genotype difference (P < 0.05); # between-genotypes difference (P < 0.05)
NOS3 rs2070744 genotype
No main effects for genotype or training modality were found (Fig. 4B). We found a tendency for a NOS3 genotype × exercise modality interaction (P = 0.07, ηp2 = 0.05), where percentage change in MVIC force was greater in CC + TC genotype following RT compared to the change in V̇O2peak in CC + TC (post hoc, P = 0.01, d = 0.84) and TT (post hoc, P = 0.03, d = 0.77) genotypes following END, and compared to the change in MVIC force in TT homozygotes following RT (post hoc, P = 0.04, Fig. 4B).
VEGFA rs2010963 genotype
There was a VEGFA genotype × exercise modality interaction (P = 0.007, ηp2 = 0.09) regarding percentage changes in MVIC force following RT and V̇O2peak after END, and a main effect for exercise modality (P = 0.03) (Fig. 4C). Post hoc t tests showed that the percentage change in MVIC force following RT was greater than the change in V̇O2peak following END in GG homozygotes only (i.e. intra-genotype differences in the adaptation to RT vs. END, paired t-test, P = 0.005, d = 0.98). Post hoc t tests showed that MVIC force improved after RT in both CC + CG (paired t-test, P < 0.001, d = 0.60) and GG (paired t-test, P < 0.001, d = 0.70) genotypes but the change in MVIC force was greater in GG homozygotes compared to CC + CG genotypes (independent t-test, P = 0.04, d = 0.75), and tended to be greater than the change in V̇O2peak after END in CC + CG genotypes (independent t-test, P = 0.07, d = 0.66). Further post hoc t tests revealed that V̇O2peak improved after END in both CC + CG (P < 0.001, d = 0.55) and GG (P = 0.002, d = 0.41) genotypes, with no difference between both genotypes (P = 0.11, d = 0.59). Furthermore, a post hoc power analysis revealed that this VEGFA genotype × exercise modality interaction had a power of 0.823.
Discussion
To the best of our knowledge, this work represents the first cross-over design study in humans, which directly compares the effects of short-term (i.e. four weeks’) endurance (END) versus resistance (RT) exercise training on cardiovascular fitness and muscle strength in the same group of individuals, and which subsequently links the intra-individual variability in the adaptations to RT vs. END to genetic variation. Although intra-individual adaptations to RT and END were unrelated (Fig. 3), VEGFA rs2010963 GG homozygotes adapted better to RT (in terms of strength gains) than they did to END (in terms of V̇O2peak improvements). VEGFA GG homozygotes also increased their MVIC force more than VEGFA C-allele carriers after RT. Thus, we have provided evidence that VEGFA rs2010963 genotype is associated with both intra- and inter-individual variation in the adaptation to RT and END in healthy young men (Fig. 4). These novel findings suggest that individuals of a certain genotype adapt better to one form of chronic exercise compared to another. Thus, depending on the training objective (e.g. strength gains or increased cardiovascular fitness), these findings suggest that exercise may soon be prescribed on an individual basis to optimise health/physical performance benefits.
Our RT and END programmes each lasted four weeks, which is sufficient time to induce gains in muscle strength (Moritani and deVries 1979; Seynnes et al. 2007; Buckthorpe et al. 2015) and cardiovascular fitness (Dunham and Harms 2012; Murias et al. 2016; Dawson et al. 2021). In our cross-over designed study, we observed an increase in MVIC force after RT but not END, while END improved V̇O2peak but not MVIC force, which is in concordance with a 12-week cross-over study (Marsh et al. 2020). Importantly, after our three-week washout period, both MVIC force (following RT) and V̇O2peak (following END) did not differ from pre-training values, thus demonstrating that the three-week detraining period was sufficient for post-training values to return to baseline (Fig. 2). Regarding the first aim of our study, we found that individual adaptations to both modes of exercise training were unrelated (Fig. 3). In other words, a high response to one exercise training modality did not relate to the adaptation to the other exercise modality. This observation reinforces a previous study, which compared both modes of exercise after a longer training duration (i.e. 12 weeks) in pairs of male and female same-sex monozygotic and dizygotic twins (Marsh et al., 2020). Despite substantial differences in methodology in the study by Marsh et al. (2020) (e.g. RT intensity; mix of upper- and lower-body exercises; 1-RM test to determine maximal strength; END incorporated both running and cycling; and V̇O2peak was performed on a treadmill), both studies demonstrated that intra-individual differences in the adaptation to RT and END were unrelated, whether the training was performed over just four weeks or for three months.
A unique aspect of our study was to explore whether SNPs within specific genes were associated with intra-individual differences in the adaptations to RT versus END. Interestingly, having two VEGFA rs2010963 G-alleles appeared to facilitate a more favourable adaptation to RT than END, while also providing an advantage over C-allele carriers regarding RT (Fig. 4). While both neural and muscle morphological adaptations contribute to strength gains following RT (Del Vecchio et al. 2019), a significant proportion of strength gains remains unexplained (Erskine et al. 2010b). Importantly, more than 80% force produced by a skeletal muscle fibre is transmitted laterally to the extracellular matrix (ECM) (Ramaswamy et al. 2011), and a RT-induced increase in this force transfer (e.g. by effectively splitting the single muscle fibre up into multiple force-generating units) may help to explain the larger gains in muscle strength than size typically seen following RT studies (Erskine et al. 2011). Matrix metalloproteinases (MMPs) play a crucial role in remodelling of the ECM by degrading various collagenous and non-collagenous ECM proteins to maintain tissue homeostasis (Medley et al. 2003), thus potentially influencing lateral force transmission within the muscle. The genes encoding MMPs have previously been associated with several musculoskeletal injuries (Posthumus et al. 2012). Specifically, several SNPs within the matrix metallopeptidase 3 (MMP3) gene have been associated with the risk of chronic Achilles tendinopathy (Gibbon et al. 2017) and anterior cruciate ligament rupture (Lulińska-Kuklik et al. 2019). More recently, we showed that academy soccer players with at least one copy of the MMP3 rs679620 T-allele had more severe injuries, and those with both copies of the VEGFA rs2010963 C-allele were 10 times more likely to be injured than GG homozygotes (Hall et al. 2021). This latter finding is likely linked to VEGFA rs2010963 C-allele carriers having a higher circulating VEGF-A concentration (Schneider et al. 2008). As VEGF-A upregulates MMP3 expression (Wang and Keiser 1998), which compromises ECM homeostasis (Wang and Keiser 1998; Hall et al. 2021), this may prevent VEGFA C-allele carriers from forming as many lateral attachments in response to loading. Thus, it is possible that this hypothetical impaired adaptation to RT may explain the smaller strength gains seen in our C-allele carriers compared to GG homozygotes, as well as the ability of our GG homozygotes to preferentially adapt to RT more than END.
While this is the first study to investigate VEGFA genotype with regards to inter- and intra-individual adaptations to RT and END, the ACTN3 rs1815739 SNP has been investigated in numerous studies regarding an association with the inter-individual adaptation to RT (Clarkson et al. 2005; Delmonico et al. 2007; Gentil et al. 2011; Pereira et al. 2013; Erskine et al. 2014) or END (Silva et al. 2015; Papadimitriou et al. 2019; Romero-Blanco et al. 2020), but this is the first time this SNP has been investigated with regards to the intra-individual adaptations to both RT and END in the same participants. Regarding the adaptation to RT alone, previous studies having reported either a beneficial effect of the R-allele (Delmonico et al. 2007; Gentil et al. 2011; Pereira et al. 2013), the X-allele (Clarkson et al. 2005), or no association (Erskine et al. 2014), while the adaptation to END alone is equally unclear (Silva et al. 2015; Papadimitriou et al. 2019; Romero-Blanco et al. 2020). Differences in study design (including population investigated, training intensity, volume and duration) may provide some explanation for the equivocal findings but our within groups cross-over design allowed us to investigate a different question, i.e. if individuals with one or two R-alleles adapted better to RT than END, and whether XX homozygotes (α-actinin-3 deficient) would adapt better to END than RT. Although we did not observe any genotype × exercise modality interaction, there was a non-significant tendency (P = 0.07) for a genotype effect, suggesting ACTN3 XX homozygotes tended to adapt better than R-allele carriers to both END and RT. Since elite endurance athletes have a higher frequency distribution of the ACTN3 XX genotype (Yang et al. 2003), and ACTN3 XX homozygotes have a greater baseline and improvement in V̇O2peak compared to individuals with ACTN3 RX and RR genotypes (Kikuchi et al. 2016), a tendency for a change in V̇O2peak following END in XX homozygotes was perhaps unsurprising. However, a tendency for XX homozygotes to adapt better to RT compared to R-allele carriers was unexpected, especially considering the greater composition of type I skeletal muscle fibres in XX homozygotes (Vincent et al. 2007) and the smaller, weaker, and less powerful characteristics of type I compared to type II skeletal muscle fibres (Gilliver et al. 2009). In an earlier study, however, young untrained women of ACTN3 XX genotype demonstrated a greater improvement in MVIC strength after RT compared to their RR counterparts (Clarkson et al. 2005), while in another study, older women of ACTN3 RR genotype increased power after RT more than their female XX homozygote counterparts (Delmonico et al. 2007). Nevertheless, it should be emphasised that our tendency for a main effect was non-significant, and this lack of association between ACTN3 genotype and adaptation to RT is in accordance with our previous work, where we demonstrated differences in muscle size, strength and power between XX homozygotes and R-allele carriers in the untrained state but, crucially, no genotype-dependent difference in the trainability to RT (Erskine et al. 2014). Thus, in contrast to young (Clarkson et al. 2005) and older (Delmonico et al. 2007) women (raising the possibility of a sex- and ageing-specific ACTN3 genotype interaction with RT adaptation), we have shown in two independent studies that the ACTN3 rs1815739 SNP does not appear to affect the adaptation to RT in young, healthy men.
The third and final SNP we investigated in this study was NOS3 rs2070744, which has previously been associated with elite power athlete status (Gómez-Gallego et al. 2009) and superior sprint/power performance (Murtagh et al. 2020). These associations may be due to reduced promoter activity of the NOS3 C-allele (Miyamoto et al. 2000), inhibiting NOS3 transcription and limiting NO production (Nakayama et al. 2000). As NO can augment myoblast fusion (Long et al. 2006), this SNP has the potential to influence muscle size and therefore strength and power. However, our study is the first to investigate an association of this SNP with the intra-individual adaptations to RT and END in terms of changes in strength and V̇O2peak, respectively, in the same homogenous group of individuals. Although not statistically significant (P = 0.07), there was a tendency for a genotype × exercise modality interaction, suggesting that C-allele carriers had the capacity to adapt better to RT than they did to END, and also better to RT than their TT homozygote counterparts. These findings are contrary to our hypothesis but, as part of this same project, we recently reported an association of this SNP with the intra-individual adaptations to RT and END in terms of vascular function (Dawson et al. 2021). We found that NOS3 C-allele carriers tended to show an improvement in vascular function after RT but not END, which we speculated may be linked to transmural pressure, thought to control vascular function during RT (Atkinson et al. 2015). This may activate NOS3 transcription independently of shear stress, which is thought to regulate vascular function during END. Thus, the mode of exercise (RT over END) may have countered the reduced promoter activity of the C-allele on NOS3 transcription. Although our study was not able to elucidate how this effect on vascular function may be related to the tendency for a similar C-allele advantage regarding strength gains following RT, it is possible that both are related to transmural pressure that is specific to RT, i.e. influencing endothelial function directly and muscle strength indirectly (the latter potentially by increasing myoblast fusion within the vascularised muscle).
Limitations
To investigate the intra-individual differences in the adaptations to RT vs. END, we used a robust cross-over design study. However, several limitations are acknowledged, which could limit the generalisation of our results to other populations. For example, we decided to focus on healthy, young men to keep our group homogeneous (thus, maximising the signal-to-noise ratio), but it is not known whether we would have seen similar results in women, or older/diseased individuals. Furthermore, although our sample size (n = 30) was large for a cross-over training intervention study, it may be considered modest for a genetics study. Nonetheless, it was larger than our a priori estimated minimal sample size (n = 28) required to detect a genotype × training modality interaction and, after controlling for multiple comparisons, we found significant associations with one of the three SNPs we investigated. Moreover, a post hoc power analysis revealed that our VEGFA genotype × training modality interaction achieved 82% power, thus confirming that our study was indeed statistically powered to detect two-way interactions. Nevertheless, independent groups should attempt to replicate our findings in larger cohorts from the same and different populations. Finally, our candidate gene approach isolated three SNPs of interest, while genome-wide association studies (GWAS) are able to investigate hundreds or thousands of SNPs simultaneously. However, unlike GWAS, our approach was hypothesis-driven and based on the evidence that these SNPs have previously been associated with exercise training adaptations and/or elite athlete status. Further, our methodology might be considered more appropriate for smaller, laboratory-controlled studies with precise phenotype measures such as this one, while GWAS technology is considerably more expensive and usually requires hundreds of samples.
Conclusion
The intra-individual adaptations to short-term RT and END in young, healthy men varied considerably but were unrelated. A variant of the VEGFA gene was linked to both the intra- and inter-individual adaptation to RT and END, i.e. VEGFA rs2010963 GG homozygotes improved their maximum strength after RT more than their cardiovascular fitness after END, and more than their VEGFA C-allele carrier counterparts’ adaptations to RT. This study is the first to provide evidence that genetic variation might help explain the intra-individual adaptation to short-term RT and END in terms of genotype-specific adaptations in strength and cardiovascular fitness, respectively. As muscular strength and cardiovascular fitness are both independent risk factors associated with all-cause mortality, these findings have important implications for personalising exercise prescription to optimise health.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- ACTN3 :
-
α-Actinin-3 gene
- 1-RM:
-
Single repetition maximum
- ANOVA:
-
Analysis of variance
- CPET:
-
Cardiopulmonary exercise testing
- ECM:
-
Extracellular matrix
- END:
-
Endurance training
- eNOS:
-
Endothelial nitric oxide synthase
- FDR:
-
False discovery rate
- HR:
-
Heart rate
- HRmax :
-
Maximal heart rate
- IKD:
-
Isokinetic dynamometer
- KE:
-
Knee extension
- MMPs:
-
Matric metalloproteinases
- MVIC:
-
Maximum voluntary isometric contraction
- NO:
-
Nitric oxide
- NOS3 :
-
Nitric oxide synthase 3 gene
- RER:
-
Respiratory exchange ratio
- RT:
-
Resistance training
- SNP:
-
Single-nucleotide polymorphism
- SS:
-
Steady-state
- VEGFA :
-
Vascular endothelial growth factor-A gene
- V̇O2 :
-
Oxygen uptake
- V̇O2peak :
-
Peak oxygen uptake
References
Atkinson CL, Carter HH, Naylor LH, Dawson EA, Marusic P, Hering D, Schlaich MP, Thijssen DH (1985) Green DJ (2015) Opposing effects of shear-mediated dilation and myogenic constriction on artery diameter in response to handgrip exercise in humans. J Appl Physiol 119:858–864
Bakeman R (2005) Recommended effect size statistics for repeated measures designs. Behav Res Methods 37:379–384
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc Series B (methodol) 57:289–300
Booth FW, Ruegsegger GN, Toedebusch RG, Yan Z (2015) Endurance exercise and the regulation of skeletal muscle metabolism. Prog Mol Biol Transl Sci 135:129–151
Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Pérusse L, Leon AS (1985) Rao DC (1999) Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol 87:1003–1008
Buckthorpe M, Erskine RM, Fletcher G, Folland JP (2015) Task-specific neural adaptations to isoinertial resistance training. Scand J Med Sci Sports 25:640–649
Campos GE, Luecke TJ, Wendeln HK, Toma K, Hagerman FC, Murray TF, Ragg KE, Ratamess NA, Kraemer WJ, Staron RS (2002) Muscular adaptations in response to three different resistance-training regimens: specificity of repetition maximum training zones. Eur J Appl Physiol 88:50–60
Clarkson PM, Devaney JM, Gordish-Dressman H, Thompson PD, Hubal MJ, Urso M, Price TB, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL (1985) Hoffman EP (2005) ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women. J Appl Physiol 99:154–163
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Publishers, Hillsdale, NJ
Dai J, Rabie AB (2007) VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res 86:937–950
Dawson EA, Sheikhsaraf B, Boidin M, Erskine RM, Thijssen DHJ (2021) Intra-individual differences in the effect of endurance versus resistance training on vascular function: a cross-over study. Scand J Med Sci Sports 31(8):1683–1692
Del Coso J, Moreno V, Gutiérrez-Hellín J, Baltazar-Martins G, Ruíz-Moreno C, Aguilar-Navarro M, Lara B, Lucía A (2019) ACTN3 R577X genotype and exercise phenotypes in recreational marathon runners. Genes (basel) 10:413
Del Vecchio A, Casolo A, Negro F, Scorcelletti M, Bazzucchi I, Enoka R, Felici F, Farina D (2019) The increase in muscle force after 4 weeks of strength training is mediated by adaptations in motor unit recruitment and rate coding. J Physiol 597:1873–1887
Delmonico MJ, Kostek MC, Doldo NA, Hand BD, Walsh S, Conway JM, Carignan CR, Roth SM, Hurley BF (2007) Alpha-actinin-3 (ACTN3) R577X polymorphism influences knee extensor peak power response to strength training in older men and women. J Gerontol A Biol Sci Med Sci 62:206–212
Dunham C, Harms CA (2012) Effects of high-intensity interval training on pulmonary function. Eur J Appl Physiol 112:3061–3068
Erskine RM, Jones DA, Maffulli N, Williams AG, Stewart CE, Degens H (2011) What causes in vivo muscle specific tension to increase following resistance training? Exp Physiol 96:145–155
Erskine RM, Jones DA, Williams AG, Stewart CE, Degens H (2010a) Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur J Appl Physiol 110:1117–1125
Erskine RM, Jones DA, Williams AG, Stewart CE, Degens H (2010b) Resistance training increases in vivo quadriceps femoris muscle specific tension in young men. Acta Physiol (oxf) 199:83–89
Erskine RM, Williams AG, Jones DA, Stewart CE (1985) Degens H (2012) Do PTK2 gene polymorphisms contribute to the interindividual variability in muscle strength and the response to resistance training? A Preliminary Report. J Appl Physiol 112:1329–1334
Erskine RM, Williams AG, Jones DA, Stewart CE, Degens H (2014) The individual and combined influence of ACE and ACTN3 genotypes on muscle phenotypes before and after strength training. Scand J Med Sci Sports 24:642–648
Gentil P, Pereira RW, Leite TK, Bottaro M (2011) ACTN3 R577X polymorphism and neuromuscular response to resistance training. J Sports Sci Med 10:393–399
Gibbon A, Hobbs H, van der Merwe W, Raleigh SM, Cook J, Handley CJ, Posthumus M, Collins M, September AV (2017) The MMP3 gene in musculoskeletal soft tissue injury risk profiling: a study in two independent sample groups. J Sports Sci 35:655–662
Gilliver SF, Degens H, Rittweger J, Sargeant AJ, Jones DA (2009) Variation in the determinants of power of chemically skinned human muscle fibres. Exp Physiol 94:1070–1078
Gómez-Gallego F, Ruiz JR, Buxens A, Artieda M, Arteta D, Santiago C, Rodríguez-Romo G, Lao JI, Lucia A (2009) The -786 T/C polymorphism of the NOS3 gene is associated with elite performance in power sports. Eur J Appl Physiol 107:565–569
Haff GG, Ruben RP, Lider J, Twine C, Cormie P (2015) A comparison of methods for determining the rate of force development during isometric midthigh clean pulls. J Strength Cond Res 29:386–395
Hall ECR, Baumert P, Larruskain J, Gil SM, Lekue JA, Rienzi E, Moreno S, Tannure M, Murtagh CF, Ade JD, Squires P, Orme P, Anderson L, Brownlee TE, Whitworth-Turner CM, Morton JP, Drust B, Williams AG, Erskine RM (2021) The genetic association with injury risk in male academy soccer players depends on maturity status. Scand J Med Sci Sports 32:338–350
Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM (2005) Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 37:964–972
Kikuchi N, Miyamoto-Mikami E, Murakami H, Nakamura T, Min SK, Mizuno M, Naito H, Miyachi M, Nakazato K, Fuku N (2016) ACTN3 R577X genotype and athletic performance in a large cohort of Japanese athletes. Eur J Sport Sci 16:694–701
Long JH, Lira VA, Soltow QA, Betters JL, Sellman JE, Criswell DS (2006) Arginine supplementation induces myoblast fusion via augmentation of nitric oxide production. J Muscle Res Cell Motil 27:577–584
Lulińska-Kuklik E, Rahim M, Moska W, Maculewicz E, Kaczmarczyk M, Maciejewska-Skrendo A, Ficek K, Cieszczyk P, September AV, Sawczuk M (2019) Are MMP3, MMP8 and TIMP2 gene variants associated with anterior cruciate ligament rupture susceptibility? J Sci Med Sport 22:753–757
Marsden PA, Heng HH, Scherer SW, Stewart RJ, Hall AV, Shi XM, Tsui LC, Schappert KT (1993) Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem 268:17478–17488
Marsh CE, Thomas HJ, Naylor LH, Scurrah KJ, Green DJ (2020) Fitness and strength responses to distinct exercise modes in twins: Studies of Twin Responses to Understand Exercise as a THerapy (STRUETH) study. J Physiol 598:3845–3858
Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ (2003) Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ Res 92:1254–1261
Miyamoto Y, Saito Y, Nakayama M, Shimasaki Y, Yoshimura T, Yoshimura M, Harada M, Kajiyama N, Kishimoto I, Kuwahara K, Hino J, Ogawa E, Hamanaka I, Kamitani S, Takahashi N, Kawakami R, Kangawa K, Yasue H, Nakao K (2000) Replication protein A1 reduces transcription of the endothelial nitric oxide synthase gene containing a -786T–>C mutation associated with coronary spastic angina. Hum Mol Genet 9:2629–2637
Moritani T, deVries HA (1979) Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med 58:115–130
Murias JM, Edwards JA, Paterson DH (2016) Effects of short-term training and detraining on VO2 kinetics: faster VO2 kinetics response after one training session. Scand J Med Sci Sports 26:620–629
Murtagh CF, Brownlee TE, Rienzi E, Roquero S, Moreno S, Huertas G, Lugioratto G, Baumert P, Turner DC, Lee D, Dickinson P, Lyon KA, Sheikhsaraf B, Biyik B, O’Boyle A, Morgans R, Massey A, Drust B, Erskine RM (2020) The genetic profile of elite youth soccer players and its association with power and speed depends on maturity status. PLoS ONE 15:e0234458
Nakayama M, Yasue H, Yoshimura M, Shimasaki Y, Ogawa H, Kugiyama K, Mizuno Y, Harada E, Nakamura S, Ito T, Saito Y, Miyamoto Y, Ogawa Y, Nakao K (2000) T(-786)–> C mutation in the 5’-flanking region of the endothelial nitric oxide synthase gene is associated with myocardial infarction, especially without coronary organic stenosis. Am J Cardiol 86:628–634
Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P (1989) Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol 59:310–319
Neufer PD (1989) The effect of detraining and reduced training on the physiological adaptations to aerobic exercise training. Sports Med 8:302–320
Papadimitriou ID, Eynon N, Yan X, Munson F, Jacques M, Kuang J, Voisin S, North KN, Bishop DJ (2019) A “human knockout” model to investigate the influence of the α-actinin-3 protein on exercise-induced mitochondrial adaptations. Sci Rep 9:12688
Pereira A, Costa AM, Izquierdo M, Silva AJ, Bastos E, Marques MC (2013) ACE I/D and ACTN3 R/X polymorphisms as potential factors in modulating exercise-related phenotypes in older women in response to a muscle power training stimuli. Age (dordr) 35:1949–1959
Posthumus M, Collins M, van der Merwe L, O’Cuinneagain D, van der Merwe W, Ribbans WJ, Schwellnus MP, Raleigh SM (2012) Matrix metalloproteinase genes on chromosome 11q22 and the risk of anterior cruciate ligament (ACL) rupture. Scand J Med Sci Sports 22:523–533
Prior SJ, Hagberg JM, Paton CM, Douglass LW, Brown MD, McLenithan JC, Roth SM (2006) DNA sequence variation in the promoter region of the VEGF gene impacts VEGF gene expression and maximal oxygen consumption. Am J Physiol Heart Circ Physiol 290:H1848-1855
Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA (2011) Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589:1195–1208
Romero-Blanco C, Artiga-González MJ, Gómez-Cabello A, Vila-Maldonado S, Casajús JA, Ara I, Aznar S (2020) Strength and endurance training in older women in relation to ACTN3 R577X and ACE I/D polymorphisms. Int J Environ Res Public Health 17:1236
Schneider BP, Radovich M, Sledge GW, Robarge JD, Li L, Storniolo AM, Lemler S, Nguyen AT, Hancock BA, Stout M, Skaar T, Flockhart DA (2008) Association of polymorphisms of angiogenesis genes with breast cancer. Breast Cancer Res Treat 111:157–163
Seto JT, Quinlan KG, Lek M, Zheng XF, Garton F, MacArthur DG, Hogarth MW, Houweling PJ, Gregorevic P, Turner N, Cooney GJ, Yang N, North KN (2013) ACTN3 genotype influences muscle performance through the regulation of calcineurin signaling. J Clin Invest 123:4255–4263
Seynnes OR, de Boer M, Narici MV (2007) Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training. J Appl Physiol 102:368–373
Silva MS, Bolani W, Alves CR, Biagi DG, Lemos JR Jr, da Silva JL, de Oliveira PA, Alves GB, de Oliveira EM, Negrão CE, Krieger JE, Dias RG, Pereira AC (2015) Elimination of influences of the ACTN3 R577X variant on oxygen uptake by endurance training in healthy individuals. Int J Sports Physiol Perform 10:636–641
Thomis MA, Beunen GP, Maes HH, Blimkie CJ, Van Leemputte M, Claessens AL, Marchal G, Willems E, Vlietinck RF (1998) Strength training: importance of genetic factors. Med Sci Sports Exerc 30:724–731
Trapé ÁA, Rodrigues JAL, Ferezin LP, Ferrari GD, Lizzi E, de Moraes VN, da Silva RF, Zago AS, Brazo-Sayavera J, Bueno Júnior CR (2021) NOS3 Polymorphisms can influence the effect of multicomponent training on blood pressure, nitrite concentration and physical fitness in prehypertensive and hypertensive older adult women. Front Physiol 12:566023
Vincent B, De Bock K, Ramaekers M, Van den Eede E, Van Leemputte M, Hespel P, Thomis MA (2007) ACTN3 (R577X) genotype is associated with fiber type distribution. Physiol Genomics 32:58–63
Wang H, Keiser JA (1998) Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells: role of flt-1. Circ Res 83:832–840
Yang N, MacArthur DG, Gulbin JP, Hahn AG, Beggs AH, Easteal S, North K (2003) ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet 73:627–631
Zmijewski P, Cięszczyk P, Ahmetov II, Gronek P, Lulińska-Kuklik E, Dornowski M, Rzeszutko A, Chycki J, Moska W, Sawczuk M (2018) The NOS3 G894T (rs1799983) and -786T/C (rs2070744) polymorphisms are associated with elite swimmer status. Biol Sport 35:313–319
Author information
Authors and Affiliations
Contributions
MB drafted the manuscript. All authors contributed to the interpretation of results, revising the manuscript and approved the final version of the manuscript and agree to be accountable for all aspects of the work. RE, ED and DT conceived and designed the study. RE and ED completed data collection, while RE and MB completed data analysis. This study was performed at Liverpool John Moores University, with the exercise interventions being conducted at the School of Sport and Exercise Sciences. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Communicated by Shuhua Xu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boidin, M., Dawson, E.A., Thijssen, D.H.J. et al. VEGFA rs2010963 GG genotype is associated with superior adaptations to resistance versus endurance training in the same group of healthy, young men. Mol Genet Genomics 298, 119–129 (2023). https://doi.org/10.1007/s00438-022-01965-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-022-01965-4