Abstract
Introduced species have a major impact on freshwater ecosystems, particularly on islands. Numerous fish species have been introduced in Corsica (Mediterranean island, southern France) as part of planned programs or clandestinely. The introduction of non-native freshwater fish species can have a range of impacts on the recipient ecosystem, including through the co-introduction of its pathogens. A sample of introduced perch Perca fluviatilis Linnaeus, 1758 from the artificial reservoir of Padula was examined following a report of parasites by an angler. The analyses revealed the occurrence of Eustrongylides sp. (Nematoda) and Clinostomum complanatum (Digenea), two zoonotic parasites in P. fluviatilis. Both parasites are reported for the first time in France. Eustrongylides sp. and C. complanatum may have been introduced with their fish intermediate hosts or through their final bird hosts. The occurrence of the two parasites raises concerns from both a veterinary and human health perspective as they can use a wide range of amphibians as intermediate hosts and can be acquired in humans through the consumption of raw or undercooked fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Southern and Western Europe are a hotspot for biological invasions, with more than a quarter of total fish diversity being non-native species (Leprieur et al. 2008). Corsica is a small island in the Northwestern Mediterranean off Southern France and Western Italy, home to a particular fish fauna composed of only four native species and naturally lacking the Cypriniformes reported from the Ibero-Franco-Italian region (Roule 1933; Changeux 1998). The fish diversity in Corsica has however been artificially increased by the man-mediated introductions of more than 20 non-native fish species, the majority of them for recreational fishing purposes (Roche and Mattei 1997; Roché 2001). The first introduced species was the mosquitofish Gambusia holbrooki Girard, 1859 in an attempt to control mosquitoes (malaria control). Then, the brook trout Salvelinus fontinalis (Mitchill, 1814) and domestic brown trout Salmo trutta Linnaeus, 1758 were introduced in several mountain lakes. Afterwards, several species, e.g., the rudd Scardinius erythrophthalmus (Linnaeus, 1758), the carp Cyprinus carpio Linnaeus, 1758, the pikeperch Sander lucioperca (Linnaeus, 1758), the tench Tinca tinca (Linnaeus, 1758), and the roach Rutilus rutilus (Linnaeus, 1758), were introduced into several artificial reservoirs and spread by anglers to rivers and others reservoirs (Roché and Mattei 1997; Roché 2001). The rainbow trout Oncorhynchus mykiss (Walbaum, 1792) is regularly released in reservoirs and lower watercourses. The study of parasites carried by non-native hosts is of great importance as they may infect native host species, a phenomenon termed parasite spillover, as well as being transmitted to humans in the case of zoonotic parasites (Daszak et al. 2000; Prenter et al. 2004; Chai et al. 2005; Lymbery et al. 2014; Chalkowski et al. 2018; Eiras et al. 2018).
The European perch Perca fluviatilis Linnaeus, 1758 was introduced in Corsica outside the framework of any planned program and from an unknown source. Its presence was first noted in 1984 during the first emptying of the Uspidali reservoir. The species was then transferred to three second-category reservoirs in the eastern plain of Corsica (namely Peri, Alzitone, and Tepe-Rosse) and now forms sustainable populations in artificial habitats, i.e., several reservoirs (the four aforementioned ones plus Codole, Tolla, and Padula) and disused gravel quarries (Roché and Mattei 1997; Roché 2001; Fleury and Le Mesle 2022). In such habitats, this species is caught by recreational anglers and regularly consumed. Recently, the occurrence of live parasites was reported by recreational anglers in freshly caught P. fluviatilis from the Padula reservoir. There is no record of transfer of P. fluviatilis to Padula reservoir and its introduction in this ecosystem is believed to be the work of individual recreational anglers. In this reservoir, P. fluviatilis is known to occur since at least 2020. Parasites of P. fluviatilis have never been examined in Corsica.
The aim of this study was to determine whether the European perch population of the Padula reservoir can host zoonotic parasites, using morphological and molecular data. Their origin and potential impact on local fauna and human health are discussed.
Material and methods
Padula Reservoir (0.25 km2 surface area, 15 m maximum depth) (Fig. 1) is an artificial reservoir on the Furmicaiola stream, located in the north of the island at an altitude of 60 m above sea level, and administered by the Office d’Equipement Hydraulique de Corse (OEHC—Corsica water resources office). It was commissioned in 1992 and serves the purposes of irrigation and drinking water supply (Colonna 2021). To our knowledge, the fish population in Padula consists of P. fluviatilis, S. lucioperca, C. carpio, S. erythrophthalmus, the largemouth black bass Micropterus salmoides (Lacepède, 1802), and a few rainbow trout O. mykiss. Amphibians are also reported from the reservoir, including two endemic Urodela: the Corsican mountain newt Euproctus montanus (Savi, 1838) and the Corsican fire salamander Salamandra corsica Savi, 1838 according to the National Inventory of Natural Heritage (Inventaire du Patrimoine Naturel – INPN) database managed by the Muséum national d’Histoire naturelle (MNHN) and the French Agency of Biodiversity (OFB) (https://inpn.mnhn.fr/espece/cd_nom/59; https://inpn.mnhn.fr/espece/cd_nom/701817). Several water and piscivorous birds visit the reservoir, e.g., the grey heron Ardea cinerea Linnaeus, 1758, the little egret Egretta garzetta (Linnaeus, 1766), and the black-crowned black heron Nycticorax nycticorax (Linnaeus, 1758) (Table 1).
Localization of Corsica in the Mediterranean Sea and of the sampling site. Diamonds are the major cities in Corsica, the red asterisk is the sampling locality (Padula Reservoir), perch icons are the localities in which the occurrence of Perca fluviatilis has been reported according to Roché and Mattei (1997), Roché (2001), as well as Fleury and Le Mesle (2022)
Ten specimens of P. fluviatilis (total length 208 ± 36 (116–248 mm), total weight 123 ± 47 (16–172 g)) were sampled by a recreational angler on 07/06/2023. Fish were brought back dead to the laboratory in an insulated bag by the angler, through the Angling Federation of Corsica (FDAAPPMA2). Fish were dissected and the body surface, opercular cavity, abdominal cavity, liver, swimbladder, and muscle tissue were thoroughly examined. However, neither the digestion (McGladdery 1986) nor the incubation (Shamsi and Suthar 2016) methods were used. As a result, an eventual underestimation of parasite abundance cannot be excluded, especially for Clinostomum, which has been reported in the skull and brain of its fish hosts (Aghlmandi et al. 2018; Shamsi et al. 2021). Parasites were recovered and preserved in either 70% or 96% ethanol for further optic and molecular identification. For scanning electron microscopic (SEM) examination, several randomly selected specimens were fixed in 2.5% glutaraldehyde (in 0.1 M sodium cacodylate buffer at pH 7.2), dehydrated through a graded ethanol series (30%, 50%, 70%, 90%, and 100%), then dried in an Emitech K850 (Quorum Technologies, Laughton, UK) critical point dryer using CO2. After mounting, the specimens were coated with platinum in a Q150T-ES sputter coater (Quorum Technologies, Laughton, UK) before examination with a Regulus 8230 scanning electron microscope (Hitachi, Tokyo, Japan) at an accelerating voltage of 2 kV. Parasite indices were calculated following the terminology of Bush et al. (1997).
Molecular identifications were done on randomly selected individuals in order to support the morphological determinations. DNA extraction, amplification, and Sanger sequencing were performed by Eurofins Genomics (Ebersberg, Germany). Both Nematoda and Digenea parasites were identified using the nuclear internal transcribed spacer (ITS) rDNA (ITS1, 5.8S, and ITS2) with the same primers 81_f 5′-GTAACAAGGTTTCCGTAGGTGAA-3′ (Gustinelli et al. 2010) and ITS2.S_r 5′-CCTGGTTAGTTTCTTTTCCTCCGC-3′ (Matthews and Cribb 1998), following Mazzone et al. (2019). For Nematoda, the mitochondrial cytochrome oxidase subunit 1 (COI) marker was also amplified using H7005 5′‒ACNACRTARTANGTRTCRTG‒3′ and L6625 5′‒TGRTTYTTYGGNCAYCC‒3′ primers (Hafner et al. 1994). DNA amplification was performed by PCR in a final 20 µl volume containing 10 µl of GoTAQ-HotStart Green MasterMix from Promega (Madison WI, USA), 4.5 µl of each of the two primers at 10 pM, and 1 µl of extracted DNA. After denaturation for 2 min at 95 °C, the PCR was run for 35 cycles of 1 min, 95 °C; 30 s, 50 °C; 1 min 30 s, 72 °C, before a final extension for 10 min at 72 °C and conserved at 4 °C on a Biometra Tadvanced thermocycler (Biometra GmbH, Göttingen, Germany). Forward and reverse sequences were assembled and cleaned with Geneious Prime ® 2020.2.4 (https://www.geneious.com). Obtained sequences were deposited in GenBank (accession numbers from PP888044-PP888052, PP888083-PP888092 and PP887879-PP887887), and were first blasted through the NCBI platform (https://blast.ncbi.nlm.nih.gov/) in order to have a preliminary identification. When several sequences were available in GenBank, molecular analyses were carried out. Sequences were aligned using MEGA X (Kumar et al. 2018) and the MUSCLE algorithm (Edgar 2004) with a molecular dataframe of reference from previous studies (Lazarova et al. 2006; Caffara et al. 2011; Xiong et al. 2013; Sereno-Uribe et al. 2013; Maleki et al. 2018; Li et al. 2018; Nitta and Ishikawa 2019; Locke et al. 2019; Pekmezci and Bolukbas 2021; Youssefi et al. 2023) (Supplementary data). JModeltest v.2.1.1 (Darriba et al. 2012) was used to estimate the best evolution model for the Bayesian phylogenetic inference analyses selected under the Bayesian Information Criterion (GTR + I for COI of Nematoda and HKY + G for ITS of Digenea). The percentage of divergence between sequences (p-distances) was calculated using MEGA X. The phylogenetic tree was constructed with MrBayes v.3.2.6 (Ronquist et al. 2012). Two independent analyses were run for 10 million generations, sampling every 200 generations. The convergence of the two analyses was checked and the tree obtained is a consensus with 10% of the trees discarded as burn-in. Two phylogenetic trees were then reconstructed on the ITS marker (145 sequences, 915 bp) for Digenea and COI marker (14 sequences, 385 bp) for Nematoda.
Median-joining networks were constructed for COI and ITS datasets from respectively Nematoda and Digenea using Network v.4.6 (Bandelt et al. 1999). A maximum parsimony algorithm was applied with the criterion “frequency > 1” to simplify the complex branching scheme and generate networks representing the most parsimonious relationships. Genetic diversity indices (haplotype diversity, number of polymorphic sites, and number of haplotypes) were calculated using DnaSP V6 (Rozas et al. 2017).
Results
Following complete parasitological examination, two different parasites were recovered from the sampled P. fluviatilis: Nematoda were morphologically identified as larvae of Eustrongylides sp. (Nematoda: Dioctophymatidae) and Digenea as metacercariae of Clinostomum (Trematoda: Clinostomidae).
Eustrongylides
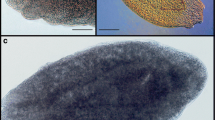
Eustrongylides larvae were found coiled in or moving through the muscles, in the swim bladder, in the abdominal cavity, and encysted in the liver. They were bright to dark red in color (Fig. 2a, b, c, d). The individuals observed in SEM showed 12 cephalic papillae arranged in 2 circles of 6 papillae each (2 lateral, 2 subdorsal, and 2 subventral with lateral papillae slightly more anterior than subdorsal and subventral papillae, Fig. 2e). Papillae of the inner circle had narrow bases and spike-like apices (Fig. 2f) and papillae of outer circle had broad bases and nipple-like apices (Fig. 2g). The anus was terminal (Fig. 2h).
Eustrongylides optic and scanning electron microscopy. a Coiled in and b free and moving through P. fluviatilis muscle. c Exiting a cyst in P. fluviatilis liver. d Exiting through the skin upon its host’s death. e Cephalic end showing both inner (arrowheads) and outer (arrows) papillae circles and the mouth. f Spike-like papillae of the inner circle with narrow base (Nb) and spike-like apex (Sa). g Nipple-like papillae of the outer circle with broad base (Bb) and nipple-like apex (Na). h Posterior end showing the terminal anus (A)
The phylogenetic tree supported the identification of Corsican specimens as members of the genus Eustrongylides. However, our COI sequences obtained from P. fluviatilis in Corsica clustered with a specimen (GenBank accession number MK013341) recovered from a pikeperch S. lucioperca (Linnaeus, 1758) sampled in Derbent Dam Lake, Turkey (Pekmezci and Bolukbas 2021) and attributed to Eustrongylides excisus Jägerskiöld, 1909 (Fig. 3a). Our sequences are grouped in five haplotypes and two haplogroups with an average divergence of 2% (Fig. 3b). These results are also in accordance with the ITS identification using BLAST where our sequences matched at 100% with the only available sequence in GenBank with a species name (KU963206) as E. excisus from a Northern pike Esox lucius Linnaeus, 1758 from Freidoonkenar, south of the Caspian Sea (Mazandaran province, Iran) (Youssefi et al. 2023).
Phylogenetic tree by Bayesian inference with a GTR + I model based on COI data (385 bp) tree of Eustrongylides, identifying 9 specimens sampled in Padula reservoir, Corsica. Numbers at nodes correspond to posterior probability values. Corresponding host species are indicated when available. No sequences were available for E. tubifex (a). Haplotypes network obtained for Eustrongylides, on the 10 sequences generated in the present study and retrieved from GenBank. Circle size is proportional to the observed haplotype frequencies and red points represent hypothetical haplotypes. Colors highlight specimens’ origin (b)
The observed prevalence was 90% as only the smallest P. fluviatilis (the only one being around 12 cm long) was found to be uninfected. The mean intensity (minimum–maximum) for Eustrongylides spp. was 29.6 ± 22.7 (3–64).
Clinostomum complanatum
Digenea metacercariae were determined as Clinostomum following Caffara et al. (2011). Clinostomum metacercariae were mainly distributed in the muscle of its hosts (75.4%) and in the opercular cavity and on gill arches (20.3%), and to a lesser extent in the abdominal cavity (2.9%) and the swimbladder (1.4%). SEM observation of excysted Clinostomum metacercariae showed an elongated body, wider in the gonadal region, with a terminal excretory pore (Fig. 4a). The oral sucker was small and surrounded by a prominent oral collar (Fig. 4b). The tegument was covered in spines along the body, sharper in the oral region (Fig. 4c) and more rounded midbody (Fig. 4d).
Clinostomum complanatum scanning electron microscopy. a Whole individual with the oral sucker (Os) and collar, ventral sucker (Vs), and excretory pore (Ep) clearly visible. b Cephalic region with the oral sucker (Os), oral collar (Oc), and sensory papillae distributed around the oral sucker. c Tegumental spines in the anterior region and d in the midbody region
The ITS phylogenetic tree includes our sequences from Corsica within the Clinostomum complanatum (Rudolphi, 1814) group with an average intrinsic distance of 0.2% (Fig. 5a). The haplotype network highlights that our sequences share the same haplotype (H1) as Italian, Romanian, Iranian, and Chinese specimens (Fig. 5b) and thus seems widely distributed in Eurasia. There was a total of 11 haplotypes, with 0.752 haplotype diversity and 14 polymorphic sites.
Phylogenetic tree by Bayesian inference with a HKY + G model based on ITS data (915 bp) of Clinostomum, identifying 10 specimens sampled in Padula reservoir, Corsica. Numbers at nodes correspond to posterior probabilities values. Corresponding host species are indicated when available (a). Haplotypes network obtained for Clinostomum complanatum, on the 48 sequences generated in the present study and retrieved from GenBank. Circle size is proportional to the observed haplotype frequencies and red points represent hypothetical haplotypes. Colors highlight specimens’ origin (b)
Morphological and molecular data corroborate the identification of Digenea parasites as C. complanatum.
The observed prevalence was 90%. The mean intensity (minimum–maximum) was 7.7 ± 3.9 (1–11) for C. complanatum.
Discussion
While molecular phylogeny enabled us to identify C. complatanatum with confidence, the lack of consensus between morphological and molecular data prevents a definitive species diagnosis in the case of Eustrongylides Nematoda. The outer circle papillae being larger than the inner circle papillae and the shape of these papillae are characteristics of Eustrongylides tubifex (Nitzsch in Rudolphi, 1819) according to the key to Eustrongylides third-stage larvae presented in Moravec (1994), while E. excisus shows similar dimensions for inner and outer circle papillae (Measures 1988; Moravec 1994; Gupta 2018; Mazzone et al. 2019). Ambiguity in morphological features may have led to confusion, especially for larval stages which have been reported not to display features allowing a reliable morphological species identification (Moravec and Nagasawa 2018; Mazzone et al. 2019). The non-exhaustiveness of molecular databases is a further issue encountered when studying Eustrongylides as the vast majority of available sequences are identified up to the genus level for these organisms. As previously noted by Shamsi et al. (2023a, b), there is no sequence available for E. tubifex and very few for E. excisus, which prevents any definite conclusion regarding the identification of the Corsican specimens. The simultaneous study of morphological features and DNA sequences of several Eustrongylides species could enable clarification of this situation. The grouping of the sequences obtained in the present study in two distinct haplogroups (H1 and H2 and H3, H4, and H5) hints at the hypothesis of two origins for this parasite, and possibly two distinct species. The occurrence of both Eustrongylides and C. complanatum are reported here for the first time in France and must be recorded in the French taxonomic register (TAXREF) of the INPN database (TAXREF 2024).
Eustrongylides prevalence in Padula reservoir (90%) is among the highest in P. fluviatilis in Europe, where reported prevalence ranges from 0.6% for E. tubifex and 13.9% for E. excisus in Bulgaria (Lake Srebarna) and 3.3%, 6.8%, and 10.0% for Eustrongylides spp. in Italy (Lake Bracciano, Trasimeno, and San Michele, respectively) to 72.0–100.0% and 94.0–100.0% for E. excisus in Ukraine (Zaporizhya Reservoir) and Moldova (Prut-Dniester interriveran), respectively (Branciari et al. 2016; Menconi et al. 2020b; Honcharov et al. 2022; Maggio et al. 2024). There does not seem to be a clear relation between Eustrongylides prevalence and the type of habitat. However, the identification of Eustrongylides larvae can be problematic and species-specific prevalence variations are not to be excluded.
The distribution of C. complanatum metacercariae mostly in the muscle and gill cavity and arches is consistent with previous reports of this parasite from fish hosts (e.g., Wang et al. 2017; Menconi et al. 2020a). The prevalence of infection was higher than that reported from Italy (14.3–21.4%, Lake Endine), Turkey (13.1% and 53.8%, Lake Sığırcı and Gala, respectively), and Czech Republic (15.0%, Morava river basin) (Kadlec et al. 2003; Çolak 2013; Soylu 2014), as well as infection intensities. As already noted by Menconi et al. (2020a), the higher prevalence observed by Soylu (2014) in Lake Gala, compared to other localities, may be attributed to its position on the avian migration route, and thus the attraction of numerous final hosts. In the same way, numerous piscivorous birds may be attracted to Padula reservoir by the nearby Biguglia lagoon, a RAMSAR wetland situated on a bird migration route (MTES 2020).
Occurrence of intermediate and final hosts
Numerous species of aquatic Oligochaeta, the usual first intermediate hosts of Eustrongylides spp., are present in Corsica (Orsini 2008). A relatively wide range of intermediate piscine hosts is known to be suitable for Eustrongylides in Europe, most notably predatory species e.g. P. fluviatilis, S. lucioperca, and the European catfish Silurus glanis Linnaeus, 1758, in Eastern Europe and more recently from Southern Europe (Italy) (Shukerova et al. 2010; Bjelić-Čabrilo et al. 2013; Branciari et al. 2016; Goncharov et al. 2018; Menconi et al. 2020b; Honcharov 2020; Guardone et al. 2021; Honcharov et al. 2022). Numerous piscivorous birds are reported as final hosts for Eustrongylides Nematoda, i.e., great cormorants Phalacrocorax carbo (Linnaeus, 1758), Anatidae (wild ducks) as well as various Ciconiformes, e.g., Egretta thula (Molina, 1803) (e.g., Honcharov et al. 2022). Moreover, Spalding and Forrester (1993) also report the occurrence of Eustrongylides from the great blue heron Ardea herodias Linnaeus, 1758. Such species, or at least congenerics, are known to occur in Corsica, where P. carbo, Ardea cinerea Linnaeus, 1758, Ardea purpurea Linnaeus, 1766, Egretta garzetta (Linnaeus, 1766) and the Anatidae Anas platyrhynchos Linnaeus, 1758, and Netta rufina (Pallas, 1773) are known to occur in the island freshwaters (Collective Losange 2017) (Table 1).
Clinostomum complanatum has been reported from several Lymnaeidae (Gastropoda) species pertaining to various genera, such as Radix euphratica (Mousson, 1874), Radix plicatula (W. H. Benson, 1842), Ampullaceana balthica (Linnaeus, 1758), Lymnaea stagnalis (Linnaeus, 1758) and Bullastra lessoni (Deshayes, 1831) (Gibson 2019; Nazarbeigy et al. 2021; Shamsi et al. 2023a, b; Wang et al. 2017). Even though there are no available data concerning malacological communities of Padula reservoir, several Lymnaeidae have been reported from Corsica: Stagnicola palustris (O. F. Müller, 1774), Galba truncatula (O. F. Müller, 1774), Peregriana peregra (O. F. Müller, 1774) and Pseudosuccinea columella (Say, 1817) (Alba et al. 2023; Dominici et al. 1996; Orsini 2008). It is thus likely that C. complanatum uses of these Lymnaeidae species as a first intermediate host in Corsica. It would be of interest to carry out a malacological survey in order to determine whether the larval stage of C. complanatum is present in the freshwater snails of the Padula reservoir. In Europe, C. complanatum is reported from several fish hosts, from numerous countries along the Danube river basin (Czechia, Hungary, Romania, Serbia, Slovakia, and Ukraine) and from Italy (Gaglio et al. 2016; Fedorčák et al. 2019; Menconi et al. 2020b). Clinostomum complanatum uses piscivorous birds final hosts either reported from Corsica or taxonomically close to hosts present on the island as its occurrence is noted in Ardea alba Linnaeus, 1758, A. purpurea, E. garzetta, A. platyrhynchos, P. carbo, and Larus gulls (Shamsi et al. 2013; El-Dakhly et al. 2018; Gibson 2019; Nazarbeigy et al. 2021) (Table 1). All the hosts needed to complete the life cycle of both Eustrongylides sp. and C. complanatum are therefore present in Corsica, and the introduction to the island of several piscine species most likely favors both these parasites’ life cycle.
As Corsica is a Mediterranean island situated on bird migration routes (Bruderer and Liechti 1999; Jourdain et al. 2007; Maggini et al. 2020), the possibility that Eustrongylides sp. and C. complanatum may have been transported with migrating birds before finding suitable fish hosts rather than being co-introduced with piscine hosts cannot be ruled out. It has been reported, for example, that Phalacrocorax carbo has possibly played a key role in the recent expansion of Eustrongylides sp. in Central and Northern Italy (Castiglione et al. 2023). However, the hypothesis of co-introduction through infected fish is also probable. Further investigations are needed to resolve this issue, if indeed it can be resolved. Furthermore, the possibility of an introduction via the first intermediate hosts cannot be ruled out. However, the lack of data concerning eventual introductions of Lymnaeidae and Oligochaeta in Corsica prevents from properly assessing this possibility.
Additionally, infection with Clinostomum or Eustrongylides were recorded in numerous amphibian species (e.g. Yildirimhan et al. 2012; Caffara et al. 2014; León-Règagnon 2019). Seven amphibian species are present in Corsica, among which three endemics. A perspective would be to conduct a parasitological examination of these organisms in order to determine if Clinostomum complanatum and/or Eustrongylides sp. could have been transferred to local amphibians. Indeed, it would be of interest as these organisms may constitute an additional reservoir for these zoonotic parasites.
Risk of food-borne Eustrongylidosis and Clinostomiasis in human
Both parasites are zoonotic as several human cases have been reported, mostly in eastern Asia (Japan, Korea) for Clinostomum and in North America (USA) for Eustrongylides (e.g., Gunby 1982; Wittner et al. 1989; Isobe et al. 1994; Chung et al. 1995; Narr et al. 1996; Hara et al. 2014; Kim et al. 2019). The infection route in human for both parasites is the consumption of either live or raw fish: Eustrongylides infections were caused either by the consumption of live bait by recreational anglers or sashimi made from unfrozen fresh fish (Gunby 1982; Wittner et al. 1989; Narr et al. 1996) and C. complanatum infections by the consumption of raw freshwater or brackish water fish (Isobe et al. 1994; Chung et al. 1995; Hara et al. 2014; Kim et al. 2019). Given the capacity of both C. complanatum and Eustrongylides spp. to infect humans, we recommend that the occurrence of these parasites in the fish of Padula reservoir should be monitored, as prevalence can change over time as was shown in Trasimeno lake, Italy (Franceschini et al. 2022). The cccurrence of the two parasites could only be analyzed in P. fluviatilis but Nematoda matching the description of Eustrongylides were also reported by a recreational angler from S. lucioperca but not from M. salmoides, both species occurring in Padula reservoir. Perca fluviatilis and S. lucioperca are prized by fishermen for their tasty meat and are commonly consumed (Fleury and Le Mesle 2022). Both the local population and tourists should be warned to avoid the raw consumption of both P. fluviatilis and S. lucioperca. In addition, the monitoring should be extended to the other reservoirs where these fish are known to occur. The occurrence of final bird hosts is another component that should be taken into account while monitoring both parasites as the population growth of the P. carbo is believed to have had a major impact on the apparent expansion of Eustrongylides sp. in Italy (Castiglione et al. 2023).
Conclusion
The parasites of the non-native P. fluviatilis were studied for the first time in the French Mediterranean island of Corsica, following the report of a recreational angler. This led to the first observation of two zoonotic parasites, the Nematoda Eustrongylides sp. and the Digenea C. complanatum. The present study is thus an example of how crucial it is that academic research and citizens communicate and work together. Clinostomum complanatum was reliably identified through the use of molecular and morphological study. Eustrongylides could not be identified at the species level, mainly because molecular databases do not cover all of this genus’ species. It is highly likely that the life cycle of both C. complanatum and Eustrongylides can be completed in Corsica as intermediate and final hots seem to be available on the island. The past introductions of numerous non-native species in Corsica may facilitate the completion of these life cycles as well as the maintenance of these parasites in Corsica. The occurrence of C. complanatum and Eustrongylides sp. is concerning from both a veterinary and human health perspective as these parasites can use a wide range of amphibians as intermediate hosts and can be acquired in humans through the consumption of raw or undercooked fish. Even if the consumption of freshly caught wild fish sushi or sashimi is not reported to be a common practice in Corsica, recreational anglers eating their catch and medical practitioners should remain vigilant. Because they potentially interact with numerous components of their ecosystem, in addition to being zoonotic, both Eustrongylides and C. complanatum are an illustration of how interconnected are human, animal, and ecosystem health, as described in the One Health framework.
Data availability
The DNA sequences generated during this study have been deposited in GenBank under the accession numbers PP888044-PP888052, PP888083-PP888092 and PP887879-PP887887.
References
Aghlmandi F, Habibi F, Afraii MA et al (2018) Infection with metacercaria of Clinostomum complanatum (Trematoda: Clinostomidae) in freshwater fishes from Southern Caspian Sea Basin. Rev Med Vet 169:147–151
Alba A, Grech-Angelini S, Vázquez AA et al (2023) Fasciolosis in the Mediterranean island of Corsica (France): insights from epidemiological and malacological investigations. Food Waterborne Parasitol 30:e00188. https://doi.org/10.1016/j.fawpar.2023.e00188
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48. https://doi.org/10.1093/oxfordjournals.molbev.a026036
Bjelić-Čabrilo O, Novakov N, Ćirković M et al (2013) The first determination of Eustrongylides excisus Jägerskiöld, 1909 — larvae (Nematoda: Dioctophymatidae) in the pike-perch Sander lucioperca in Vojvodina (Serbia). Helminthologia 50:291–294. https://doi.org/10.2478/s11687-013-0143-1
Bowdish BS (1948) Heron mortality caused by Eustrongylides ignotus. Auk 65:602–603. https://doi.org/10.2307/4080622
Branciari R, Ranucci D, Miraglia D et al (2016) Occurrence of parasites of the genus Eustrongylides spp. (Nematoda: Dioctophymatidae) in fish caught in Trasimeno Lake, Italy. Ital J Food Safety 5:6130. https://doi.org/10.4081/ijfs.2016.6130
Bruderer B, Liechti F (1999) Bird migration across the Mediterranean. Proc Int Ornithol Congr, Durban. BirdLife South Africa, Johannesburg, pp 1983–1999
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583. https://doi.org/10.2307/3284227
Caffara M, Locke SA, Gustinelli A et al (2011) Morphological and molecular differentiation of Clinostomum complanatum and Clinostomum marginatum (Digenea: Clinostomidae) metacercariae and adults. J Parasitol 97:884–891. https://doi.org/10.1645/GE-2781.1
Caffara M, Bruni G, Paoletti C et al (2014) Metacercariae of Clinostomum complanatum (Trematoda: Digenea) in European newts Triturus carnifex and Lissotriton vulgaris (Caudata: Salamandridae). J Helminthol 88:278–285. https://doi.org/10.1017/S0022149X13000151
Castiglione D, Di Maggio M, Guardone L et al (2023) Eustrongylides excisus in fish species caught in the Massaciuccoli Lake (Northwest Tuscany, Italy): implications for freshwater fish quality and public health. Food Control 153:109894. https://doi.org/10.1016/j.foodcont.2023.109894
Caudill G, Wolf D, Caudill D et al (2014) A juvenile wading-bird mortality event in urban Jacksonville, Florida, associated with the parasite Eustrongylides. Fla Field Nat 42:108–113
Chai J-Y, Darwin Murrell K, Lymbery AJ (2005) Fish-borne parasitic zoonoses: status and issues. Int J Parasitol 35:1233–1254. https://doi.org/10.1016/j.ijpara.2005.07.013
Chalkowski K, Lepczyk CA, Zohdy S (2018) Parasite ecology of invasive species: conceptual framework and new hypotheses. Trends Parasitol 34:655–663. https://doi.org/10.1016/j.pt.2018.05.008
Changeux T (1998) Insular characteristics of freshwater fish communities in the island of Corsica, comparison with French continental coastal rivers. Ital J Zool 65:305–311. https://doi.org/10.1080/11250009809386838
Chung DI, Moon CH, Kong HH et al (1995) The first human case of Clinostomum complanatum (Trematoda: Clinostomidae) infection in Korea. Korean J Parasitol 33:219. https://doi.org/10.3347/kjp.1995.33.3.219
Çolak H (2013) Metazoan parasites of fish species from Lake Sığırcı (Edirne, Turkey). Turk J Vet Anim Sci 37:200–205. https://doi.org/10.3906/vet-1202-28
Collective Losange (2017) Corse : reconnaître toutes les espèces. Artémis édtions, Chamalière, France
Colonna F (2021) Les conséquences du changement climatique sur les ressources en eau et le peuplement piscicole des cours d’eau de Corse. PhD Dissertation, Université de Corse Pascal Paoli
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and high-performance computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science 287:443–449. https://doi.org/10.1126/science.287.5452.443
Dominici JL, Oviedo JA, Mas Coma S et al (1996) Liver fluke disease (Fasciola hepatica, Trematoda) in Corsica. Altitudinal distribution of intermediate host. Lymnaea truncatula. Vie Milieu 46:379
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Eiras JC, Pavanelli GC, Takemoto RM, Nawa Y (2018) Fish-borne nematodiases in South America: neglected emerging diseases. J Helminthol 92:649–654. https://doi.org/10.1017/S0022149X17001006
El-Dakhly KhM, El-Nahass E, Uni S et al (2012) Levels of infection of gastric nematodes in a flock of great cormorants (Phalacrocorax carbo) from Lake Biwa, Japan. J Helminthol 86:54–63. https://doi.org/10.1017/S0022149X11000046
El-Dakhly KM, Hussein N, El-Nahass E-S (2018) Occurrence of helminths in the great cormorants, Phalacrocorax carbo, in Qena. Egypt J Adv Vet Res 8:6–11
Fedorčák J, Šmiga Ľ, Kutsokon I et al (2019) Parasitic infection of Cobitis elongatoides Băcescu & Mayer, 1969 by zoonotic metacercariae Clinostomum complanatum (Rudolphi, 1814). J Fish Dis 42:1677–1685. https://doi.org/10.1111/jfd.13097
Fleury J-P, Le Mesle H (2022) Animaux de corse. Albiana, Ajaccio
Franceschini R, Guardone L, Armani A et al (2022) Five-years management of an emerging parasite risk (Eustrongylides sp., Nematoda) in a fishery supply chain located on Trasimeno Lake (Italy). Food Control 136:108858. https://doi.org/10.1016/j.foodcont.2022.108858
Gaglio G, Reina V, Caffara M et al (2016) Risk of introduction of Clinostomum complanatum (Digenea: Clinostomidae) to Sicily through use of Cobitis bilineata (Canestrini, 1865) as live baits. Bull Eur Assoc Fish Pathol 36:105–110
Gibson D (2019) Clinostomum complanatum (Rudolphi, 1814) Braun, 1899. In: WoRMS. https://www.marinespecies.org/aphia.php?p=taxdetails&id=725814. Accessed 10 Oct 2023
Gohardehi S, Fakhar M, Madjidaei M (2013) Avian schistosomes and human cercarial dermatitis in a wildlife refuge in Mazandaran Province, Northern Iran. Zoonoses Public Health 60:442–447. https://doi.org/10.1111/zph.12020
Goncharov SL, Soroka NM, Pashkevich IY et al (2018) Infection of predatory fish with larvae of Eustrongylides excisus (Nematoda, Dioctophymatidae) in the delta of the Dnipro River and the Dnipro-Buh estuary in Southern Ukraine. Vestn Zool 52:137–144. https://doi.org/10.2478/vzoo-2018-0015
Guardone L, Ricci E, Susini F et al (2021) First detection of Eustrongylides excisus (Nematoda: Dioctophymatidae) in big-scale sand smelt (Atherina boyeri) from the lake Massaciuccoli (Northwest Tuscany, Italy): implications for public health and seafood quality. Food Control 120:107517. https://doi.org/10.1016/j.foodcont.2020.107517
Gunby P (1982) One worm in the minnow equals too many in the gut. J Am Med Assoc 248:163. https://doi.org/10.1001/jama.1982.03330020011004
Gupta N (2018) Light and scanning electron microscopic studies on Eustrongylides exciscus larvae (Nematoda: Dioctophmida) from Channa punctatus Bloch from India. Pak J Zool 51:159–166. https://doi.org/10.17582/journal.pjz/2019.51.1.159.166
Gustinelli A, Caffara M, Florio D et al (2010) First description of the adult stage of Clinostomum cutaneum Paperna, 1964 (Digenea: Clinostomidae) from grey herons Ardea cinerea L. and a redescription of the metacercaria from the Nile tilapia Oreochromis niloticus niloticus (L.) in Kenya. Syst Parasitol 76:39–51. https://doi.org/10.1007/s11230-010-9231-5
Hafner MS, Sudman PD, Villablanca FX et al (1994) Disparate rates of molecular evolution in cospeciating hosts and parasites. Science 265:1087–1090. https://doi.org/10.1126/science.8066445
Hara H, Miyauchi Y, Tahara S, Yamashita H (2014) Human laryngitis caused by Clinostomum complanatum. Nagoya J Med Sci 76:181
Honcharov SL (2020) Prevalence of the nematodes Eustrongylides exisus Jägerskiöld, 1909, – larvae (Nematoda: Dioctophymatidae) infection in the Rutilisrutilis, linnaeus 1758 and the seasonal dynamics of the infection in the waters of the Dnipro-Buh estuari in southern Ukraine. Sci Messen LNU Vet Med Biotech Ser Vet Sci 22:31–38
Honcharov SL, Soroka NM, Halat MV et al (2022) Distribution of the nematodes of the genus Eustrongylides (Nematoda, Dioctophymatidae) in the world. Regul Mech Biosyst 13:73–79. https://doi.org/10.15421/022210
Isobe A, Kinoshita S, Hojo N et al (1994) The 12th human case of Clinostomum sp. Infection in Japan. Jpn J Parasitol 43:193–198
Jourdain E, Gauthier-Clerc M, Bicout D, Sabatier P (2007) Bird migration routes and risk for pathogen dispersion into western Mediterranean wetlands. Emerg Infect Dis 13:365. https://doi.org/10.3201/eid1303.060301
Kadlec D, Šimková A, Jarkovskỳ J, Gelnar M (2003) Parasite communities of freshwater fish under flood conditions. Parasitol Res 89:272–283. https://doi.org/10.1007/s00436-002-0740-2
Kavetska KM, Pilarczyk B, Królaczyk K (2012) Stomach nematodes of wild ducks (subfamily Anatinae) wintering in the North-Western Poland. Bull Vet Inst Pulawy 56:27–31. https://doi.org/10.2478/v10213-012-0005-5
Kim H, Cho S-W, Oh H, Byeon HK (2019) A case of unexpected Clinostomum complanatum Infection initially presenting as foreign body in pharynx. Korean J Parasitol 57:175–177. https://doi.org/10.3347/kjp.2019.57.2.175
Kumar S, Stecher G, Li M et al (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lazarova SS, Malloch G, Oliveira CMG et al (2006) Ribosomal and mitochondrial DNA analyses of Xiphinema americanum-Group Populations. J Nematol 38:404–410
León-Règagnon V (2019) Helminths of The Eurasian Marsh Frog, Pelophylax Ridibundus (Pallas, 1771) (Anura: Ranidae), from the Shiraz Region, Southwestern Iran. Helminthologia 56:261–268. https://doi.org/10.2478/helm-2019-0022
Leprieur F, Beauchard O, Blanchet S et al (2008) Fish invasions in the world’s river systems: when natural processes are blurred by human activities. PLoS Biol 6:e28. https://doi.org/10.1371/journal.pbio.0060028
Li BF, Liu X-H, Ge H-L et al (2018) The discovery of Clinostomum complanatum metacercariae in farmed Chinese sucker, Myxocyprinus asiaticus. Aquaculture 495:273–280. https://doi.org/10.1016/j.aquaculture.2018.05.052
Locke LN (1961) Heron and egret losses due to verminous peritonitis. Avian Dis 5:135–138. https://doi.org/10.2307/1587612
Locke SA, Caffara M, Barčák D et al (2019) A new species of Clinostomum Leidy, 1856 in East Asia based on genomic and morphological data. Parasitol Res 118:3253–3265. https://doi.org/10.1007/s00436-019-06536-y
Lymbery AJ, Morine M, Kanani HG et al (2014) Co-invaders: the effects of alien parasites on native hosts. Int J Parasitol Parasites Wildl 3:171–177. https://doi.org/10.1016/j.ijppaw.2014.04.002
Maggini I, Cardinale M, Sundberg JH et al (2020) Recent phenological shifts of migratory birds at a Mediterranean spring stopover site: species wintering in the Sahel advance passage more than tropical winterers. PLoS ONE 15:e0239489. https://doi.org/10.1371/journal.pone.0239489
Maggio MD, Coltraro M, Tinacci L et al (2024) Mapping the occurrence of Eustrongylides spp. in fish species caught from six lakes in Central Italy (Tuscany and Latium regions): implications for local fishery supply chains. Heliyon 10:e30733. https://doi.org/10.1016/j.heliyon.2024.e30733
Maleki L, Heidari H, Ghaderi E, Rostamzadeh J (2018) Occurrence and description of Clinostomumcomplanatum (Rudolphi, 1819) metacercariae in freshwater fishes from Gheshlagh basin, West of Iran. Iran J Anim Biosyst 14:91–103. https://doi.org/10.22067/ijab.v14i2.74577
Matthews D, Cribb TH (1998) Digenetic trematodes of the genus Clinostomum Leidy, 1856 (Digenea: Clinostomidae) from birds of Queensland, Australia, including C. wilsoni n. sp. from Egretta intermedia. Syst Parasitol 39:199–208. https://doi.org/10.1023/A:1005982530560
Mazzone A, Caffara M, Gustinelli A et al (2019) Morphological and molecular characterization of larval and adult stages of Eustrongylides excisus (Nematoda: Dioctophymatoidea) with histopathological observations. J Parasitol 105:882–889. https://doi.org/10.1645/19-44
McGladdery SE (1986) Anisakis simplex (Nematoda: Anisakidae) infection of the musculature and body cavity of Atlantic herring (Clupea harengus harengus). Can J Fish Aquat Sci 43:1312–1317. https://doi.org/10.1139/f86-164
Measures L (1988) Epizootiology, pathology, and description of Eustrongylides tubifex (Nematoda: Dioctophymatoidea) in fish. Can J Zool 66:2212–2222. https://doi.org/10.1139/z88-329
Menconi V, Manfrin C, Pastorino P et al (2020) First report of Clinostomum complanatum (Trematoda: Digenea) in European perch (Perca fluviatilis) from an Italian Subalpine Lake: a risk for public health? Int J Environ Res Public Health 17:1389. https://doi.org/10.3390/ijerph17041389
Menconi V, Riina MV, Pastorino P et al (2020) First occurrence of Eustrongylides spp. (Nematoda: Dioctophymatidae) in a Subalpine Lake in Northwest Italy: new data on distribution and host range. Int J Environ Res Public Health 17:4171. https://doi.org/10.3390/ijerph17114171
Monteiro CM, Amato JFR, Amato SB (2011) Helminth parasitism in the Neotropical cormorant, Phalacrocorax brasilianus, in Southern Brazil: effect of host size, weight, sex, and maturity state. Parasitol Res 109:849–855. https://doi.org/10.1007/s00436-011-2311-x
Moravec F (1994) Parasitic nematodes of freshwater fishes of Europe, 1st edn. Kluwer Academic Publishers, Dordrecht
Moravec F, Nagasawa K (2018) Rhabdochona angusticaudata sp. n. (Nematoda: Rhabdochonidae) from the Japanese eel Anguilla japonica, and new records of some other nematodes from inland fishes in Japan. Folia Parasitol 65:016. https://doi.org/10.14411/fp.2018.016
MTES (Ministère de la Transition Ecologique et Solidaire) (2020) Fiche RAMSAR: etang de Biguglia. https://www.zones-humides.org/etang-de-biguglia-0. Accessed 9 Apr 2024
Narr LL, O’Donnell JG, Libster B et al (1996) Eustrongylidiasis - a parasitic infection acquired by eating live minnows. J Osteopath Med 96:400–400. https://doi.org/10.7556/jaoa.1996.96.7.400
Nazarbeigy M, Halajian A, Amadi A (2021) Checklist of digenean trematodes of Iran. Vet Parasitol Reg Stud Reports 24:100571. https://doi.org/10.1016/j.vprsr.2021.100571
Nitta M, Ishikawa T (2019) Metacercariae of Clinostomum complanatum (Platyhelminthes: Trematoda: Clinostomidae), a parasite of the northern medaka, Oryzias sakaizumii (Beloniformes: Adrianichthyidae), from Yamagata Prefecture Japan. Biogeography 21:17–21
Orsini A (2008) Les cours d’eau de corse: caractérisation et anthropisation. Habilitation à Diriger des Recherche Dissertation, Université de Corse Pascal Paoli
Pekmezci GZ, Bolukbas CS (2021) Morphological and molecular characterization of Eustrongylides excisus larvae (Nematoda: Dioctophymatidae) in Sander lucioperca (L.) from Northern Turkey. Parasitol Res 120:2269–2274. https://doi.org/10.1007/s00436-021-07187-8
Pinto RM, Barros LA, Tortelly L et al (2004) Prevalence and pathology of helminths of ciconiiform birds from the Brazilian swamplands. J Helminthol 78:259–264. https://doi.org/10.1079/JOH2004243
Prenter J, MacNeil C, Dick JTA, Dunn AM (2004) Roles of parasites in animal invasions. Trends Ecol Evol 19:385–390. https://doi.org/10.1016/j.tree.2004.05.002
Roché B, Mattei J (1997) Les espèces animales introduites dans les eaux douces de corse. Bull Fr Pêche Piscic 344/345:233–239. https://doi.org/10.1051/kmae:1997025
Roché B (2001) Atlas des poissons d’eau de douce de corse. Direction Régionale de l'Environnement de Corse, Bastia
Roffe TJ (1988) Eustrongylides sp. epizootic in young common egrets (Casmerodius albus). Avian Dis 32:143–147. https://doi.org/10.2307/1590964
Ronquist F, Teslenko M, van der Mark P et al (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Roule L (1933) Le peuplement des cours d’eau de la Corse en poissons. Bull Fr Piscic 63:61–62. https://doi.org/10.1051/kmae:1933007
Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC et al (2017) DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol 34:3299–3302. https://doi.org/10.1093/molbev/msx248
Rusconi A, Prati P, Bragoni R et al (2022) Occurrence of Eustrongylides excisus (Nematoda: Dioctophymatidae) in European perch (Perca fluviatilis) and great cormorant (Phalacrocorax carbo) in Lake Annone, northern Italy. J Parasitol 108:209–216. https://doi.org/10.1645/20-175
Sereno-Uribe AL, Pinacho-Pinacho CD, García-Varela M, Pérez-Ponce de León G (2013) Using mitochondrial and ribosomal DNA sequences to test the taxonomic validity of Clinostomum complanatum Rudolphi, 1814 in fish-eating birds and freshwater fishes in Mexico, with the description of a new species. Parasitol Res 112:2855–2870. https://doi.org/10.1007/s00436-013-3457-5
Shamsi S, Suthar J (2016) A revised method of examining fish for infection with zoonotic nematode larvae. Int J Parasitol 227:13–16. https://doi.org/10.1016/j.ijfoodmicro.2016.03.023
Shamsi S, Halajian A, Tavakol S et al (2013) Pathogenicity of Clinostomum complanatum (Digenea: Clinostomidae) in piscivorous birds. Res Vet Sci 95:537–539. https://doi.org/10.1016/j.rvsc.2013.06.018
Shamsi S, Scott D, Zhu X et al (2021) Wild fish as reservoirs of parasites on Australian Murray cod farms. Aquaculture 539:736584. https://doi.org/10.1016/j.aquaculture.2021.736584
Shamsi S, Banfield A, Francis N et al (2023) Occurrence of digenean parasites in freshwater snails in the Murrumbidgee catchment area, Australia. Food Waterborne Parasitol 32:e00202. https://doi.org/10.1016/j.fawpar.2023.e00202
Shamsi S, Francis N, Masiga J et al (2023) Occurrence and characterisation of Eustrongylides species in Australian native birds and fish. Food Waterborne Parasitol 30:e00189. https://doi.org/10.1016/j.fawpar.2023.e00189
Shukerova S, Kirin D, Hanzelová V (2010) Endohelminth communities of the perch, Perca fluviatilis (Perciformes, Percidae) from Srebarna Biosphere Reserve, Bulgaria. Helminthologia 47:99–104. https://doi.org/10.2478/s11687-010-0016-9
Soylu E (2014) Metazoan parasites of fish species from Lake Gala (Edirne, Turkey). Ege J Fish Aquat Sci 31:187–193. https://doi.org/10.12714/egejfas.2014.31.4.03
Spalding MG (1990) Antemortem diagnosis of Eustrongylidosis in wading birds (Ciconiiformes). Col Waterbirds 13:75–77. https://doi.org/10.2307/1521425
Spalding MG, Forrester DJ (1993) Pathogenesis of Eustrongylides ignotus (Nematoda: Dioctophymatoidea) in Ciconiiformes. J Wildl Dis 29:250–260. https://doi.org/10.7589/0090-3558-29.2.250
Sutherland M, Shamsi S, Phalen DN, Macwhirter PJ (2018) Eustrongylides induced verminous coelomitis in an Australian darter (Anhinga novaehollandiae). Eolophus 2:33–36
Švažas S, Chukalova N, Grishanov G et al (2011) The role of great cormorant (Phalacrocorax carbo sinensis) for fish stock and dispersal of helminthes parasites in the Curonian Lagoon area. Vet Zootech 55:79–85
TAXREF (ed) (2024) TAXREF v17.0, référentiel taxonomique pour la France. PatriNat (OFB-CNRS-MNHN), Muséum national d'Histoire naturelle, Paris. https://inpn.mnhn.fr/telechargement/referentielEspece/taxref/17.0/menu. Accessed 5 Jan 2024
Wang M-L, Chen H-Y, Shih H-H (2017) Occurrence and distribution of yellow grub trematodes (Clinostomum complanatum) infection in Taiwan. Parasitol Res 116:1761–1771. https://doi.org/10.1007/s00436-017-5457-3
Wittner M, Turner JW, Jacquette G et al (1989) Eustrongylidiasis — a parasitic infection acquired by eating sushi. N Engl J Med 320:1124–1126. https://doi.org/10.1056/NEJM198904273201706
Xiong F, Li WX, Wu SG et al (2013) Molecular phylogeny and host specificity of the larval Eustrongylides (Nematoda: Dioctophmidae) from freshwater fish in China. J Parasitol 99:137–144. https://doi.org/10.1645/GE-3163.1
Yildirimhan HS, Sümer N, Incedoğan S, Bursey CR (2012) Helminth parasites of the lemon-yellow tree frog, Hyla savignyi (Hylidae), from Turkey. Turk J Zool 36:171–184. https://doi.org/10.3906/zoo-1006-9
Youssefi MR, Tabaripour R, Hosseini M (2023) Molecular characterisation and histopathological study of Eustrongylidesexcisus nematode in the northern pike (Esoxlucius). Bulg J Vet Med 26:81–88. https://doi.org/10.15547/bjvm.2392
Acknowledgements
The authors are grateful to Ghislain Delbreil, a recreational angler, for reporting the occurrence of parasites and providing the samples.
Funding
The present study was partially funded as a doctoral fellowship of the University of Corsica Pasquale Paoli and the Culletivittà di Corsica granted to Anaïs Esposito (no grant number available). This research is part of the GERHYCO interdisciplinary project dedicated to water management, ecology, and hydro-ecosystem services in an insular context, and it was financially supported by the Culletivittà di Corsica.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by A.E., G.P.J.D., V.H., P.J-.A., J.F., and Y.Q. The first draft of the manuscript was written by A.E. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
As the individuals analyzed were obtained already dead as a result of an opportunity sampling conducted by a recreational angler as part of their leisure activity, in compliance with the recreational angling legislation (Arrêté Préfectoral n°2B-2023–03-09–00003 available at: https://www.haute-corse.gouv.fr/contenu/telechargement/6879/56039/file/recueil-2b-2023-03-004-recueil-des-actes-administratifs-special.pdf), no approval by an ethics committee was required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no financial or proprietary interest in any material discussed in this article.
Additional information
Handling Editor: Julia Walochnik.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esposito, A., Denys, G.P.J., Haÿ, V. et al. Unregulated introduced fish (Perca fluviatilis Linnaeus, 1758) is host to zoonotic parasites in a small Mediterranean island. Parasitol Res 123, 247 (2024). https://doi.org/10.1007/s00436-024-08264-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00436-024-08264-4