Abstract
Anisakidosis is a foodborne zoonotic infection induced by members of the family Anisakidae via the consumption of raw or undercooked fish such as sushi and sashimi. Identifying anisakid larval species is critical for the epidemiology and diagnosis of diseases caused by them. This study aimed at identifying Anisakis larvae collected from marine fish in Egyptian waters based on morphological characteristics and molecular analysis. Thirty marine fish coral trout, Plectropomus areolatus, were collected from Hurghada, Red Sea, Egypt, to investigate larval nematodes of the genus Anisakis. The larvae were detected encapsulated in the peritoneal cavity and muscle of the fish host. This examination revealed that anisakid larvae naturally infected 19 fish specimens with a prevalence of 63.33% and a mean intensity of 4.1 ± 0.40. Most of them (68 larvae: 71.57%) were found in the musculature. Morphological and morphometric analyses using light and scanning electron microscopy revealed a head region with a prominent boring tooth, inconspicuous lips, and a characteristic protruded cylindrical mucron. All larvae in this study possessed the same morphology as Anisakis Larval type I. Molecular analysis based on ITS region using maximum likelihood and Bayesian phylogenetic methods confirmed them as Anisakis typica. This is the first study to identify A. typica larvae from the commercial fish coral trout P. areolatus in Egyptian waters using morphological and molecular methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish-borne zoonotic nematodes are a significant public health problem worldwide (Chai et al. 2005; Mattiucci et al. 2018; Bao et al. 2019). Anisakid nematodes are cosmopolitan parasites of marine organisms, with marine mammals serving as their definitive hosts, and the third-stage larvae infect marine fishes as paratenic hosts (Nieuwenhuizen and Lopata 2013; Pozio 2013). Anisakidosis is a zoonotic disease acquired through eating raw or insufficiently cooked fish infected with third-stage larvae of the genera Anisakis, Pseudoterranova, and Contracaecum (Mattiucci and Nascetti 2008; Bao et al. 2017; Shamsi et al. 2018). Infections by anisakid larvae (L3) are widespread in Europe and eastern Asia, commonly in Japan, Peru, and Chile, as a result of increasing the consumption of raw or undercooked fish and seafood (Chai et al. 2005; Sohn et al. 2015; Eiras et al. 2018; Martínez-Rojas et al. 2021). In humans, these parasites can cause severe gastroenteritis (Shamsi and Butcher 2011; Baptista-Fernandes et al. 2017). They also contribute to allergic reactions (Audicana and Kennedy 2008; Lopata and Lehrer 2009; Jabbar et al. 2012). These reactions are caused by chemical compounds found in fish meat, which are produced by the parasite (Pozio 2013; Ivanovic et al. 2017; Kochanowski et al. 2020).
The first report of infection by Anisakis larvae in humans documented by Van Thiel et al. (1960) raised awareness of fish-borne parasitic diseases. These nematodes penetrate the stomach wall and cause gastrointestinal symptoms, including stomach pain, vomiting, and nausea (Smith and Wootten 1978; Valls et al. 2005; Ivanović et al. 2017; Mattiucci et al. 2018). Furthermore, Anisakis infection has been linked to an increased risk of gastrointestinal cancer or tumors (Garcia-perez et al. 2015; Corcuera et al. 2018).
Anisakid larvae can be identified at the genus level by the morphological features of anterior and posterior regions; these larvae are further divided into four groups, types I–IV, based on the length of the ventriculus and the presence or lack of a tail spine (mucron). Anisakis type I larvae have a longer ventriculus and a distinct mucron, including A. simplex s.s., A. pegreffii, A. typica, A. ziphidarum, A. berlandi, and A. nascettii, while types II–IV larvae have a shorter ventriculus and no mucron, including A. physeteris, A. brevispiculata, and A. paggiae respectively (Murata et al. 2011; Mattiucci et al. 2018; Shamsi 2021).
Specific morphological identification of L3 anisakid larvae is relatively challenging due to the low development of the organs at this stage and the absence of remarkable features (Tunya et al. 2020). Generally, the difficulty of identifying nematodes depends on several parameters, such as the small size of the worms, the great diversity of nematodes found in a sample, and the absence of specific morphological traits (Farjallah et al. 2008; Seesao et al. 2017). Furthermore, they are retrieved from frozen fish or patient organs and tissues (Dick et al. 1991; Abou-Rahma et al. 2016). Therefore, molecular techniques, such as DNA sequencing, are the most reliable methods to identify anisakid larvae (Lin et al. 2007; Tunya et al. 2020). Several studies have demonstrated the utility of nuclear and mitochondrial DNA markers such as large ribosomal DNA (28S), internal transcribed spacer (ITS), and cytochrome c oxidase subunit 1 (cox1) and subunit 2 (cox2) for the identification of ascaridoid worms (Li et al. 2017; Zhang et al. 2018; Guo et al. 2021). In Egypt, some previous studies recorded Anisakis spp. from different marine fish exhibiting various prevalences reached 76.7% of Anisakis sp. (type I) in European sea bass Dicentrarchus labrax (Morsy et al. 2012), 35% of Anisakis sp. (type II) in greater lizard fish Saurida undosquamis (Morsy et al. 2015), 36.6% of Anisakis simplex s.s. from European Hake Merluccius merluccius lessepsianus (Abou-Rahma et al. 2016), 19.05% and 42.86) of Anisakis sp. (type I) in smoked herring and frozen mackerel fish (Arafa et al. 2019), 87.1% and 83.3% of Anisakis simplex s.s. in Atlantic herring and Mediterranean horse mackerel, whereas 42.8% of Anisakis typica in Atlantic mackerel (Mostafa et al. 2020).
The study aimed to contribute to the epidemiology and molecular identification of Anisakis spp. infecting commercially important fish in Egypt.
Materials and methods
The current study was performed following guidelines approved by the Cairo University Institutional Animal Care and Use Committee (CU-IACUC) and approved under the relevant document (No. CU/I/F/32/19).
A total of 30 coral trout, P. areolatus (F: Serranidae), were purchased from local fish markets in Hurghada city, Red Sea. Fish specimens were dissected and the body cavity, digestive tract, visceral organs examined for nematode parasites. The musculature was sliced into thin slivers (1.0–2.0-mm thick) and then visually inspected for parasites under white light. Larvae were removed from the surrounding host tissues with the aid of a stereomicroscope, noting the site of infection then washed in physiological saline, counted and preserved in 70% ethanol until use.
Nematodes were cleared in lactophenol for morphological studies (Pritchard and Kruse 1982). The total number of larvae were analyzed (N = 25), and the number of measured larvae were (N = 5) for every parameter. The identification was based on the main characteristics of larval anisakids, such as anteriorly located boring tooth or lips, the length of the ventriculus, the postanal tail’s shape, and the presence or absence of a terminal mucron (Pinto et al. 1994; Rocka 2004). All body measurements were taken with ocular micrometers and photomicrographic images were obtained with a LEICA DM 750 microscope. Scanning electron microscopy (SEM) was used to examine nematode larvae that had been fixed in a glutaraldehyde solution of 2.5%. After 24 h, samples were post-fixed in phosphate buffer containing 1% osmium tetroxide (OsO4) for 24 h, dehydrated through a graded ethanol series (50%, 60%, 70%, 80%, 90%, and 100%), and dried at 30 °C for 30 min using a critical point drier (LEICA, EM CPD300). Dried specimens were mounted on aluminum stubs with carbon tape, coated with gold, and examined with a JEOL JSM-5200 SEM (Tokyo, Japan) at an accelerating voltage 25 kV (Guo et al. 2014).
For molecular studies, Genomic DNA was extracted from individual larvae (N = 25) after being preserved in 70% ethanol using a QIAamp® DNA Mini Kit (Qiagen) following the manufacturer’s protocol. PCR amplification of the internal transcribed spacer (ITS1-5.8S-ITS2) region of ribosomal DNA (rDNA) used two universal primers NC5 (forward; 5′-GTAGGTGAACCTGCGGAAGGATCATT-3′ and NC2 (reverse; 5′- TTAGTTTCTTTTCCTCCGCT-3′) (Zhu et al. 1998). PCR reactions were performed in a total volume of 50 μl as follows: 25 μl PCR Super-Mix (Genetech) containing dNTP, MgCl2, buffer, and Taq-polymerase,1 µl of 10 Pmol of both forward and reverse primers and 3 μl parasite genomic DNA, and then it completed by 20 μl of nuclease free water. Thermocycling conditions involved an initial denaturation step at 94 °C for 5 min, then 35 cycles of denaturation step at 94 °C for 30 s, primer annealing at 58 °C for 30 s, extension at 72 °C for 30 s, and a final extension at 72 °C for 7 min (Thermal Cycler, Model FTC3/20 (TC-3000X, TECHNE, Bibby scientific, and United Kingdom) according to Costa et al. (2018) with some modifications.
PCR products were analyzed by gel electrophoresis and visualized using a UV transilluminator (Cedex 1, France). Stained DNA fragments were photographed using a gel documentation analysis system. PCR products were purified using a gel purification kit (Genedirex. Inc) and sequenced using an automated sequencer, ABI PRISM model 377 version 3.3.1 (Clinilab, Egypt). The nucleotide sequence obtained in this study was deposited in GenBank under accession number OM371077. BLAST searches were performed at the National Center for Biotechnology Information (NCBI) database (http:// www.ncbi.nlm. nih.gov) to find sequence similarities. The query sequence plus those retrieved from GenBank were aligned using Bioedit version 3.3.19.0, and phylogenetic trees were constructed using maximum likelihood method based on Kimura 2-parameter model by the MEGA software version 11.0.10 (Tamura et al. 2021) with bootstrapping of 1000 replications and a Bayesian method in BEAST software version v1.10.4 (Suchard et al. 2018). Procamallanus fulvidraconis (DQ076698) was used as an outgroup.
Results and discussion
Compared to earlier investigations in Egypt on this genus, 95 nematode specimens were found in 19 out of 30 P. areolatus investigated (63.33%), as indicated in Table 1. All obtained samples correspond to Anisakis L3. The larvae were found encapsulated in the fish’s body cavity and muscles. The mean larval intensity was 4.1 ± 0.4 (1–5 larvae/fish). Most of them were retrieved from the fish muscles (68 larvae: 71.57%), in contrast to the recent finding of Tunya et al. (2020), who isolated these Anisakis larvae primarily from the peritoneal cavity. Because humans frequently consume fish muscles, epidemiological concerns about the presence of larvae in muscles are significant, including human health, product safety, and ecological interest (Ayun et al. 2021). Furthermore, our larvae were only detected during visual inspection without incubation, in contrast to Shamsi and Suthar (2016), which reported that combining visual examination and incubation method was more effective in detecting the predominance of larvae. These parasites can emerge from internal organs and migrate to the flesh of the fish (Smith and Wootten 1975), which explains the higher prevalence of larvae in the muscles.
The morphological examination using a standard light microscope and SEM showed that the bodies of larvae were cylindrical, attenuated at both ends, and measured 18.1 ± 2.1 (16.58–19.68) mm long and 0.45 ± 0.02 (0.29–0.51) mm wide. The anterior region exhibited a prominent boring tooth, and the lips were inconspicuous. The esophagus displayed an anterior muscular part measured 1.35 ± 0.02 (1.28–1.46) mm long. The ventriculus was long and cylindrical measured 0.78 ± 0.15 (0.59–0.98) mm long and 0.17 ± 0.02 (0.12–0.22) mm wide. The excretory pore is located anteriorly. The intestine was long. The anus is located at 0.95 ± 0.15 (1.12–1.45) mm from the posterior end. Also, scanning electron microscopy showed the characteristic triradiate mouth opening surrounded by four amphids and transverse and longitudinal striations of the cuticle (Figs. 1, 2, and 3). These characteristic features of L3 larvae were identical to Anisakis larvae type I, particularly the long ventriculus and mucron at the posterior larval end (Mattiucci et al. 2018; Shamsi 2021). These larvae also displayed short rounded tails with a characteristic cylindrical bently protruded mucron measuring 0.018 ± 0.002 (0.0162–0.027) mm long, much similar to previous descriptions of Tunya et al. (2020) and Hien et al. (2021). In addition, the present species was compared, as shown in Table 2, with other previously published A. typica. Most of its body measurements were closer to those described from the Asian region rather than from Egypt which confirming this specie’s worldwide distribution.
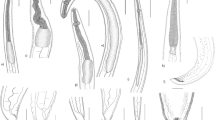
Photomicrographs of L3 Anisakis typica recovered from P. areolatus cleared in lactophenol. a Anterior region of worm showing mouth opening (MO) provided with boring tooth (BT) and long muscular esophagus (OS). b High magnification of anterior extremity indicate mouth opening (MO), boring tooth (BT), and esophagus (OS). c Posterior region showing intestine (IN) opens ventrally by anal opening (A) and ended with a cylindrical mucron (M). d High magnification of posterior extremity showing anal opening (A) and cylindrical mucron (M)
Scanning electron micrographs of L3 Anisakis typica. a, b Anterior extremity showing triangular mouth opening (MO), boring tooth (BT), and four amphids (AM). c, d Body cuticle showing longitudinal striations (LS) and transverse striations (TS). e, f Posterior extremity showing the characteristic mucron (M)
Although Tunya et al. (2020) stated that the protruded mucron of L3 larvae might be used to differentiate anisakid larvae at the species level, additional research is required to validate this identification. Various genes, including the internal transcribed spacer region (ITS), cytochrome oxidase subunits (cox1), and cox2 have been utilized to identify the nematode parasites (Zhang et al. 2018). The ITS1-5.8S-ITS2 region of ribosomal DNA is a suitable marker for nematode species identification (Abollo et al. 2003), as this region exhibits higher nucleotide sequence differences between species.
Because the Anisakis larvae collected in this study were all morphologically similar, molecular identification was carried out on 25 isolated larvae. Nucleotide sequencing of the ITS region of rDNA (ITS-1, 5.8S, and ITS-2) yielded 670 bp and deposited in GenBank under the accession number OM371077. A comparison of Anisakis larval ITS1-5.8S-ITS2 rDNA nucleotide sequences exhibited high blast scores with previously published A. typica sequences in the GenBank, as shown in (Fig. 4). The greatest genetic similarity was 99.85% to specimens from Papua New Guinea (JX648318), Indonesia (KC928261), Coasts of Taiwan and Japan (AB432908), Turkish waters (KF032062), while 99.40% to (MN420660) from Thailand. Moreover, the phylogenetic trees of ITS regions of different Anisakis species were constructed using maximum likelihood and Bayesian methods as presented in (Figs. 5 and 6). The inferred topologies significantly supported the monophyly of Anisakis spp. The two phylogenetic methods produced similar topology results in terms of clades with high support values, confirming that our Anisakis larvae (OM371077) were clustered with other A. typica a distinct clade from other Anisakis species based on 100% bootstrap value explaining the genetic relationship with those published in the GenBank and promoting us to identify the present species parasitizing P. areolatus as A. typica.
Maximum likelihood showing the phylogenetic relationships of Anisakis typica larva reported herein based on ITS of rDNA with other different species using Kimura 2-parameter model and 1000 bootstrap replications with a complete deletion. Bootstrap support values are indicated above the nodes, and Procamallanus fulvidraconis was used as an outgroup. Asterisks represent the present sample (OM371077 **)
Phylogenetic tree was constructed based on ITS region of rDNA exploring the relationships among Anisakis typica of the present study using Bayesian inference (BI) method. Bootstrap percentages are shown at the internal nodes. Procamallanus fulvidraconis was used as an outgroup. Asterisks represent the present sample (OM371077 **)
The close genetic relationship of the current A. typica from Egyptian waters, the Red Sea, with different specimens from various geographical locations, may be attributed to the widespread dispersal of A. typica and the migration of its final hosts, Cetaceans, and its larvae infecting several marine fishes around the world (Shamsi et al. 2017). Anisakis type I, particularly A. simplex (s.s.), A. pegreffii, and A. typica, have been recovered from a wide variety of fish all over the world (Ayun et al. 2021), and A. typica has been commonly found in warmer temperate and tropical seas (Mattiucci et al. 2018), with some reports from the Mediterranean coast of Gabes city in Tunisia, Australia, New Caledonia, China, Vietnam, and from Thailand (Farjallah et al. 2008; Jabbar et al. 2012; Shamsi et al. 2018; Guo et al. 2020; Tunya et al. 2020; Cheypanya et al. 2021).
Anisakiasis in humans was most frequently linked to A. simplex s.s. and A. pegreffii (Aibinu et al. 2019). But, cases of infection by A. typica were rarely recorded, and due to the lack of information regarding the illness caused by their larvae (Tunya et al. 2020), the zoonotic effect may be underestimated (Umehara et al. 2010). Furthermore, the parasite was only identified as Anisakis larval type I in the majority of human reports (Bruschi and Dupouy-Camet 2014). While molecular techniques can accurately identify species of Anisakis larvae, medical professionals don't employ them to distinguish anisakid larvae from their patients (Rahmati et al. 2020). In addition, Anisakis larvae infecting humans are frequently damaged or fragmented upon removal, preventing any attempt for identification at the genus level (Mattiucci et al. 2013), and the diagnosis of allergic anisakidosis is primarily based on non-specific serology tests (Ubeira 2014). Therefore, the majority of diagnoses assumed the larvae to be Anisakis simplex s.s. This means that the possibility of human infection by other species of Anisakis cannot be completely ruled out, although the current species was considered a non-zoonotic parasite until now. The existence of these parasites in the edible portion/muscle of the fishes posed a relatively high risk to human health when consumed. In this study, we used molecular analysis to identify Anisakis larvae based on ITS1-5.8S-ITS2 rDNA nucleotide sequences as A. typica in the marine fish coral trout, P. areolatus, a new host record from the Red Sea in Egypt.
Specimen deposition
Specimens were deposited in Zoology Department, Faculty of Science, Cairo University, Cairo, Egypt (Code No. P002).
Conclusion
Based on our detailed description utilizing light and scanning electron microscopy and advanced molecular analysis such as DNA sequencing, we were able to elucidate the precise taxonomy of the current larvae as A. typica. This study adds important information to the previous reports on the occurrence of Anisakis larvae infecting commercial fish in Egypt, which may be highly relevant to public health issues, and highlights the necessity of further research on other fishes preferred by the Egyptian population.
Change history
03 March 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00436-023-07802-w
References
Abollo E, Paggi L, Pascual S, D’Amelio S (2003) Occurrence ofrecombinant genotypes of Anisakis simplex s.s. and Anisakis pegreffii (Nematoda: Anisakidae) in an area of sympatry. Infect Genet Evol 3:175–181. https://doi.org/10.1016/s1567-1348(03)00073-x
Abou-Rahma Y, Abdel-Gaber R, Ahmed AK (2016) First record of Anisakis simplex third-stage larvae (Nematoda, Anisakidae) in European Hake Merluccius merluccius lessepsianus in Egyptian water. J Parasitol Res. 2016:1–8. https://doi.org/10.1155/2016/9609752
Aibinu IE, Smooker PM, Lopata AL (2019) Anisakis nematodes in fish and shell fish from infection to allergies. Int J Parasitol Parasites Wildl 9:384–393. https://doi.org/10.1016/j.ijppaw.2019.04.007
Arafa WM, Hassan AHA, Mahrous LN, Abdel-Ghany AE, Aboelhadid SM (2019) Occurrence and molecular characterization of zoonotic Anisakis simplex sensu stricto and Anisakis pegreffii larvae in retail-marketed fish. J Food Safety 39(5):e12682. https://doi.org/10.1111/jfs.12682
Audicana MT, Kennedy MW (2008) Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev 21:360–379. https://doi.org/10.1128/CMR.00012-07
Ayun NQ, Dewi LS, Murwantoko Setyobudi E (2021) The occurrence of Anisakis larvae on hairtail, Trichiurus lepturus caught from the Pangandaran Waters, West Java Indonesia. Biodiversitas 22(3):1378–1384. https://doi.org/10.13057/biodiv/d220339
Bao M, Pierce GJ, Pascual S, González-Muñoz M, Mattiucci S, Mladineo I, Cipriani P, Bušelic I, Strachan NJC (2017) Assessing the risk of an emerging zoo-nosis of worldwide concern: anisakiasis. Sci Rep 7:43699. https://doi.org/10.1038/srep43699
Bao M, Pierce GJ, Strachan NJC, Pascual S, González-Muñoz M, Levsen A (2019) Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis: challenges in risk assessment. Trends Food Sci Technol 86:298–310. https://doi.org/10.1016/j.tifs.2019.02.013
Baptista-Fernandes T, Rodrigues M, Castro I, Paixão P, Pinto-Marques P, Roque L, Belo S, Ferreira PM, Mansinho K, Toscano C (2017) Human gastric hyperinfection by Anisakis simplex: a severe and unusual presentation and a brief review. Int J Infect Dis 64:38–41. https://doi.org/10.1016/j.ijid.2017.08.012
Bruschi F, Dupouy-Camet J (2014) Helminth infections and their impact on global public health. Springer, Vienna, Austria
Chai JY, Murrell KD, Lymbery AJ (2005) Fish-borne parasitic zoonoses: status and issues. Int J Parasitol 35:1233–1254. https://doi.org/10.1016/j.ijpara.2005.07.013
Cheypanya V, Wongsawad Ph, Wongsawad Ch, Nantarat N (2021) Morphological study and molecular epidemiology of Anisakis larvae in mackerel fish. Asian Pac J Trop Med 14(5):214–222. https://doi.org/10.4103/1995-7645.315900
Corcuera MT, Rodríguez-Bobada C, Zuloaga J, Gómez-Aguado F, Rodríguez-Perez R, Mendizabal Á, González P, Arias-Díaz J, Caballero ML (2018) Exploring tumourigenic potential of the parasite Anisakis: a pilot study. Parasitol Res 117:3127–3136. https://doi.org/10.1007/s00436-018-6008-2
Costa A, Graci S, Cammilleri G, Buscemi MD, Collura R, Vella A, Ferrantelli V (2018) Molecular identification of Hysterothylacium Spp. In Fishes fromthe Southern Mediterranean Sea (Southern Italy). J Parasitol 104(4):398–406. https://doi.org/10.1645/16-60
Dick TA, Dixon BR, Choudhury A (1991) Diphyllobothrium, Anisakis and other fish-borne parasitic zoonoses. Southeast Asian J Trop Med Public Health 22(suppl):150–152
Eiras JC, Pavanelli GC, Takemoto RM, Nawa Y (2018) Fish-borne nematodiases in South America: neglected emerging diseases. J Helminthol 92:649–654. https://doi.org/10.1017/S0022149X17001006
Farjallah S, Slimane BD, Busi M, Paggi L, Amor N, Blel H, Said K, D’Amelio S (2008) Occurrence and molecular identification of Anisakis spp. from the North African coasts of Mediterranean Sea. Parasitol Res 102(3):371–379. https://doi.org/10.1007/s00436-007-0771-9
Garcia-perez JC, Rodríguez-Perez R, Ballestero A, Zuloaga J, Fernandez-puntero B, Arias-Díaz J, Caballero ML (2015) Previous exposure to the fish parasite Anisakis as a potential risk factor for gastric or colon adenocarcinoma. Medicine (baltimore) 94(40):1699. https://doi.org/10.1097/MD.0000000000001699
Guo YN, Xu Z, Zhang LP, Hu YH, Li L (2014) Occurrence of Hysterothylacium and Anisakis nematodes (Ascaridida: Ascaridoidea) in the Tanaka’s snailfish Liparis tanakae (Gilbert & Burke) (Scorpaeniformes: Liparidae). Parasitol Res 113(4):1289–1300. https://doi.org/10.1007/s00436-014-3767-2
Guo N, Chen H-X, Zhang L-P, Zhang J-Y, Yang L-Y, Li L (2020) Infection and molecular identification of ascaridoid nematodes from the important marine food fish Japanese threadfin bream Nemipterus japonicus (Bloch) (Perciformes: Nemipteridae) in China. Infect Genet Evol 85:104562. https://doi.org/10.1016/j.meegid.2020.104562
Guo N, Sitko J, Chen H-X, Li L (2021) Morphological and genetic characterization of Porrocaecum angusticolle (Molin, 1860) (Nematoda: Ascaridomorpha) from the common buzzard Buteo buteo (Linnaeus) (Accipitriformes: Accipitridae) in Czech Republic. Parasitol Int 83:102365. https://doi.org/10.1016/j.parint.2021.102365
Hien HV, Dung BT, Ngo HD, Doanh PN (2021) First morphological and molecular identification of third-stage larvae of Anisakis typica (Nematoda: Anisakidae) from marine fishes in Vietnamese water. J Nematol 53:1–10. https://doi.org/10.21307/jofnem-2021-010
Ivanović J, Baltić MZ, Bošković M, Kilibard N, Dokmanović M, Marković R, Janjić J, Baltić B (2017) Anisakis allergy in human. Trends Food Sci Tech 59:25–29. https://doi.org/10.1016/j.tifs.2016.11.006
Jabbar A, Asnoussi A, Norbury LJ, Eisenbarth A, Shamsi Sh, Gasser RB, Lopata AL, Beveridge I (2012) Larval anisakid nematodes in teleost fishes from Lizard Island, northern Great Barrier Reef, Australia. Mar Freshw Res 63:1283–1299. https://doi.org/10.1071/MF12211
Kochanowski M, Dabrowska J, Rozycki M, Karamon J, Sroka J, Cencek T (2020) Proteomic profiling reveals new insights into the allergomes of Anisakis simplex, Pseudoterranova decipiens, and Contracaecum osculatum. J Parasitol 106(5):572–588. https://doi.org/10.1645/19-75
Li L, Zhao J-Y, Chen H-X, Ju H-D, An M, Xu Z, Zhang L-P (2017) Survey for the presence of ascaridoid larvae in the cinnamon flounder Pseudorhombus cinnamoneus (Temminck & Schlegel) (Pleuronectiformes: Paralichthyidae). Int J Food Microbiol 241:108–116. https://doi.org/10.1016/j.ijfoodmicro.2016.10.018
Lin RQ, Dong SJ, Nie K, Wang CR, Li AX, Song HQ, Huang WY, Zhu XQ (2007) Sequence analysis of the first internal transcribed spacer of rDNA supports the existence of an intermediate Fasciola between F. hepatica and F. gigantica in main land China. Parasitol Res 101(3):813–817. https://doi.org/10.1007/s00436-007-0512-0
Lopata AL, Lehrer SB (2009) New insights into seafood allergy. Curr Opin Allergy Clin Immunol 9:270–277. https://doi.org/10.1097/ACI.0b013e32832b3e6f
Martínez-Rojas R, Mondragón-Martínez A, De-Los-Santos ER, Cruz-Neyr L, García-Candela E, Delgado-Escalante A, Sanchez-Venegasb JR (2021) Molecular identification and epidemiological data of Anisakis spp. (Nematoda: Anisakidae) larvae from Southeastern Pacific Ocean off Peru. Int J Parasitol Parasites Wildlife 16:138–144. https://doi.org/10.1016/j.ijppaw.2021.09.001
Mattiucci S, Nascetti G (2008) Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and hostparasite co-evolutionary processes. Adv Parasitol 66:47–148. https://doi.org/10.1016/S0065-308X(08)00202-9
Mattiucci S, Fazii P, de Rosa A, Paoletti M, Megna AS, Glielmo A, de Angelis M, Costa A, Meucci C, Calvaruso V, Sorrentini I, Palma G, Bruschi F, Nascetti G (2013) Anisakiasis and gastroallergic reactions associated with Anisakis pegreffii infection. Italy Emerg Infect Dis 19(3):496–499. https://doi.org/10.3201/eid1903.121017
Mattiucci S, Cipriani P, Levsen AMM, Nascetti G (2018) Molecular epidemiology of Anisakis and anisakiasis: an ecological and evolutionary road map. Adv Parasitol 99:93–263. https://doi.org/10.1016/bs.apar.2017.12.001
Morsy K, Bashtar AR, Abdel-Ghaffar F, Mehlhorn H, Quraishy SA, El-Mahdi M, Al-Ghamdi A, Mostafa N (2012) First record of anisakid juveniles (Nematoda) in the European seabass Dicentrarchus labrax (family: Moronidae), and their role as bio-indicators of heavy metal pollution. Parasitol Res 110(3):1131–1138. https://doi.org/10.1007/s00436-011-2600-4
Morsy K, Bashtar AR, Mostafa N, El Deeb S (2015) Thabet S (2015) New host records of three juvenile nematodes in Egypt: Anisakis sp. (Type II), Hysterothylacium patagonense (Anisakidae), and Echinocephalus overstreeti (Gnathostomatidae) from the greater lizard fish Saurida undosquamis of the Red Sea. Parasitol Res 114:1119–1128. https://doi.org/10.1007/s00436-014-4285-y
Mostafa E, Omar M, Shimaa S, Hassan Sh, Samir M (2020) Occurrence and molecular identification of Anisakis larval type 1 (Nematoda: Anisakidae) in marketed fish in Egypt. J Parasit Dis 44:536–545. https://doi.org/10.1007/s12639-020-01222-8
Murata R, Suzuki J, Sadamasu K, Kai A (2011) Morphological and molecular characterization of Anisakis larvae (Nematoda: Anisakidae) in Beryx splendens from Japanese waters. Parasitol Int 60:193–198. https://doi.org/10.1016/j.parint.2011.02.008
Nieuwenhuizen NE, Lopata AL (2013) Anisakis - a food-borne parasite that triggers allergic host defences. Int J Parasitol 43:1047–1057. https://doi.org/10.1016/j.ijpara.2013.08.001
Pinto RM, Vicente JJ, Noronha D, Gonçalves L, Gomes DC (1994) Helminth parasites of conventionally maintained laboratory mice. Mem Inst Oswaldo Cruz 89(1):33–40. https://doi.org/10.1590/s0074-02761994000100007
Pozio E (2013) Integrating animal health surveillance and food safety: the example of Anisakis. Rev Sci Tech Off int Epiz 32(2):487–496. https://doi.org/10.20506/rst.32.2.2246
Pritchard MH, Kruse GO (1982) The collection and preservation of animal parasites. University of Nebraska Press, Lincoln, Nebraska, p 141
Rahmati AR, Kiani B, Afshari A, Moghaddas E, Williams M, Shamsi S (2020) World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: a systematic review. Parasitol Res 119(11):3585–3594. https://doi.org/10.1007/s00436-020-06892-0
Rocka A (2004) Nematodes of the Antarctic fishes. Pol Polar Res 25(2):135–152
Seesao Y, Gay M, Merlin S, Viscogliosi E, Aliouat-Denis CM, Audebert C (2017) A review of methods for nematode identification. J Microbiol Methods 138:37–49. https://doi.org/10.1016/j.mimet.2016.05.030
Shamsi S (2021) The occurrence of Anisakis spp. in Australian waters: past, present, and future trends. Parasitol Res. 120(9):3007–3033. https://doi.org/10.1007/s00436-021-07243-3
Shamsi S, Butcher AR (2011) First report of human anisakidosis in Australia. Med J Aust 194:199–200. https://doi.org/10.5694/j.1326-5377.2011.tb03772.x
Shamsi S, Suthar J (2016) A revised method of examining fish for infection with zoonotic nematode larvae. Int J Food Microbiol 227:13–16. https://doi.org/10.1016/j.ijfoodmicro.2016.03.023
Shamsi S, Briand MJ, Justine J (2017) Occurrence of Anisakis (Nematoda: Anisakidae) larvae in unusual hosts in southern hemisphere. Parasitol Int 66(6):837–840. https://doi.org/10.1016/j.parint.2017.08.002
Shamsi S, Chen Y, Poupa A, Ghadam M, Justine J-L (2018) Occurrence of anisakid parasites in marine fishes and whales off New Caledonia.Parasitol Res.117(10):3195–3204. https://doi.org/10.1007/s00436-018-6018-0
Smith JW, Wootten R (1975) Experimental studies on the migration of Anisakis sp. larvae (Nematoda:Ascaridida) into the flesh of herring Clupea Harengus l. Int J Parasitol 5:133–136. https://doi.org/10.1016/0020-7519(75)90019-3
Smith JW, Wootten R (1978) Anisakis and anisakiasis. Adv Parasitol 16:93–163. https://doi.org/10.1016/S0065-308X(08)60573-4
Sohn WM, Na BK, Kim TH, Park TJ (2015) Anisakiasis: report of 15 gastric cases caused by Anisakis type I larvae and a brief review of Korean anisakiasis cases. Korean J Parasitol 53(4):465–470. https://doi.org/10.3347/kjp.2015.53.4.465
Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ & Rambaut A (2018) Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10 Virus Evolution 4, vey016. https://doi.org/10.1093/ve/vey016
Tamura K, Stecher G, Kumar S (2021) MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol. https://doi.org/10.1093/molbev/msab120
Tunya R, Wongsawad C, Wongsawad P, Chai JY (2020) Morphological and molecular characteristics of Anisakis typica larvae in two species of threadfin bream, Nemipterus hexodon and N. japonicus, from the Gulf of Thailand. Korean J Parasitol 58(1):15–25. https://doi.org/10.3347/kjp.2020.58.1.15
Ubeira FM (2014) Travelling with Anisakis allergens. Int Arch Allergy Immunol 163(4):243–244. https://doi.org/10.1159/000360361
Umehara A, Kawakami Y, Ooi HK, Uchida A, Ohmae H, Sugiyama H (2010) Molecular identification of Anisakis type I larvae isolated from hairtail fish off the coasts of Taiwan and Japan. Int J Food Microbiol 143:161–165. https://doi.org/10.1016/j.ijfoodmicro.2010.08.011
Valls A, Pascual CY, Esteban MM (2005) Anisakis allergy: an update. Rev Fr Allergol 45(2):108–113. https://doi.org/10.1016/j.allerg.2005.01.005
Van Thiel PH, Kuipers FC, Roskam RT (1960) A nematode parasitic to herring, causing acute abdominal syndromes in man. Trop Geogr Med 12: 97–113. https://pubmed.ncbi.nlm.nih.gov/13776308
Zhang K, Xu Z, Chen H-X, Guo N, Li L (2018) Anisakid and raphidascaridid nematodes (Ascaridoidea) infection in the important marine food-fish Lophius litulon (Jordan) (Lophiiformes: Lophiidae). Int J Food Microbiol 284:105–111. https://doi.org/10.1016/j.ijfoodmicro.2018.08.002
Zhu X, Gasser RB, Podolska M, Chilton NB (1998) Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. Int J Parasitol 28:1911–1921. https://doi.org/10.1016/S0020-7519(98)00150-7
Acknowledgements
The present study is supported by Faculty of Science, Cairo University, Egypt. The authors extend their appreciations to members of Zoology Department, Laboratory of Parasitology in helping to complete this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Section Editor: Shokoofeh Shamsi
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The authors regret that there are some errors in their article, including the mis-identification of the fish host species.
The original article has been corrected.
Highlights
• Anisakiasis is one of the most prevalent fish-borne nematode zoonosis infections caused by consuming raw or undercooked fish infected with anisakid larvae.
• The prevalence of Anisakis larvae in marine fishes is extremely concerning for human health, product safety, and the environment.
• Using molecular techniques like PCR amplification and sequencing of the internal transcribed spacer (ITS) region of rDNA, we can precisely identify anisakid larvae.
• The present anisakid larvae Anisakis typica recovered from marine fish, coral trout Plectropomus areolatus as a new host record from the Red Sea in Egypt.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mostafa, N.A., Abdel-Ghaffar, F., Fayed, H.O. et al. Morphological and molecular identification of third-stage larvae of Anisakis typica (Nematoda: Anisakidae) from Red Sea coral trout, Plectropomus areolatus. Parasitol Res 122, 705–715 (2023). https://doi.org/10.1007/s00436-022-07776-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07776-1