Abstract
This study focuses on Anisakidosis as a disease caused by a number of Anisakid larvae including Anisakis simplex, Pseudoterranova decipiens, Hysterothylacium spp. and Contracaecum spp., through assessing the clinical signs and the clinical examination of the disease in 170 Lizard head (Saurida undosquamis) and 160 European hakes (Merluccius merluccius) during the period from April 2011 to July 2012. In addition, identification of the recovered larvae morphologically and using random modified polymorphic DNA (RAPD-PCR) technique. No obvious clinical signs were observed in the examined fish. The post mortem examination revealed the presence of free or encapsulated larvae within the body cavity, on the visceral organs and in the musculature. The seasonal prevalence of Anisakid larvae was the highest in spring and summer and its lowest level was in autumn in both examined fish spp. The highest intensity of larvae was observed in the organs and viscera rather than in the musculature. RAPD technique identified the recovered larvae using four arbitrary primers. It could be concluded that Anisakid larvae can be easily identified using molecular tools by its well characterized genetic finger print and that in turn will help in its diagnosis in parallel with the traditional tools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. Introduction
Anisakids are nematodes from super family Ascaridoidea (families: Anisakidae and Raphidascarididae). Specially those belong to genera (Anisakis, Pseudoterranova, Contracecum and Hystrothylacium) are of biological and economic importance in the aquatic environment [1]. The life cycle of Anisakid nematodes involves crustaceans (e.g., Krill) as transport hosts, fishes (e.g., herring, hakes, cod) as intermediate hosts and marine mammals (Whale, Sea lions, Dolphin and Seal) as definitive hosts [2]. Although humans are accidental hosts; in which Anisakid larvae do not complete its development but may penetrate the alimentary tract and invade associated organs, causing a range of pathological effects [3, 4]. Moreover, owing to the thermo-stability of Anisakis simplex allergens, the ingestion of safely cooked fish containing dead parasites can also be potentially dangerous and can cause severe allergic reactions such as contact dermatitis and asthma [5, 6]. Anisakid larvae can be identified microscopically at genus level based on the morphology of the digestive tract and excretory system [7]. In Egypt, various species of Anisakid larvae have been reported based on the morphology [8, 9, 10]. The main target of this study was to survey the prevalence and abundance of Anisakid larvae from both European (Merluccius merluccius) and Lizard head (Saurida undosquamis) hakes; depending on both the morphological characters and the molecular genetic finger prints using random modified polymorphic DNA (RAPD-PCR) which is extremely powerful tool for routine identification of Anisakid larvae and has proved for genetically characterizing different species of Anisakid nematodes and their larval stages [11]. In addition they can estimate their genetic differentiation and relationships [12].
II. Materials and methods
A total of 330 marine fish belonging to hake fishes; 170 Lizard head (Saurida undosquamis) and 160 European hakes (Merluccius merluccius) were collected from Suez canal and Mediterranean Sea respectively on ice to the lab of Fish Diseases and Management, Benha University, Egypt, since April 2011 to July 2012, where the examination was conducted as soon as possible. Clinical and post mortem examinations were done for all the collected fish species, for determination of any lesions or abnormalities externally and internally following [13]. Each fish was dissected and the abdominal cavity, stomach, serous tissue, spleen, kidney, liver and gonads were examined by naked eyes for Anisakid larvae. Meanwhile, 10 gm of musculature were taken from around the body cavity of each fish and examined under the dissecting microscope. The collected larvae were washed in distilled water, and kept in 70% ethanol for both morphological identification and DNA extraction.
A. Morphological examination
The recovered larvae were cleared in lactophenol and permanently mounted in glycerin-gelatin. The slides were left to dry for 24 hours and examined microscopically [14]. The following morphological characteristics were measured: body width, esophagus length, ventriculus length, tail length, body length/body width, body length/esophagus length, body length/ventriculus length and body length/tail length. All measurements were made directly with an eyepiece micrometer and were given in millimeters. The larvae were then identified according to their morphological characteristic features following [15, 16, 27].
The prevalence and the intensity of anisakid larvae infestations were calculated as defined by [17].
B. DNA extraction
DNA extraction from Anisakid larvae was carried out using SDS method of [18] with few modifications. Two to five anisakid larvae (irrespective their type) were placed in an Eppendorf tube and kept in liquid nitrogen for few seconds to facilitate the rupture of cell membranes. The tissue was crushed by a pestle in lysis buffer (10 μl of SCE (sorbitol 1M, sodium citrate 0.1 M, EDTA 0.06 M), 30 μl of SDS-EDTA (SDS 1%, EDTA 0.15 M) and 10 μl of proteinase K 1%) and then incubated at 42 ºC over night. Subsequently, the DNA was purified with one phenol–chloroform–isoamyl alcohol extraction, followed by one chloroform-isoamyl alcohol extraction and then an ethanol precipitation. The precipitated pellet was re-suspended in 50 μl distilled water and kept at -20 ºC until use.
C. Polymerase chain reaction (PCR) conditions
Ten μl from DNA aliquot of each sample was mixed well to prepare the bulked DNA sample representing Anisakid larvae. This generated bulked DNA sample was used for RAPD-PCR fingerprint of Anisakids using four arbitrary primers “Table.2”. PCR was performed in 25 μl volumes tubes containing; 2.5μl DNTPs (0.5mM), 1.5μl MgCl2 (1.5mM), 2.5μl Buffer (10X), 2.5μl Primer (10 p.mol), 0.35μl Taq DNA polymerase (250 U), 2μl Template DNA (25ng) and 13.65μl H2O (DW). Amplification reactions were carried out in (Perkin Elmer Gene Amp PCR Thermocycler 2400), with the following conditions; 94ºC for 2.5 min followed by 40 cycles including a denaturation step at 94ºC for 45 sec., an annealing step at 37 ºC for 30 sec. and polymerization step at 72ºC for 2 min. with a final extension step at 72ºC for 12 min.
D. PCR product electrophoresis
A volume of 15 μl of the amplified RAPD products was loaded in each well in sub marine mini gel agarose electrophoresis apparatus (BIORAD) and standards DNA (100 bp +1.5 kb ladder DNA yielding 1500, 1000, 900, 800, 700, 600, 500,400,300,200 and 100 base pair (bp) bands as well as 50 bp ladder producing bands of 500, 450, 400, 350, 300, 250, 200, 150, 100 and 50 bp) were loaded onto a 1.2% agarose gel and separated by electrophoresis. Gel was stained with ethidium bromide (0.2μg/ml) and then the products were visualized by UV light. PCR products were photographed by gel documentation system (Gel Doc. BIORAD 2000) under UV trans-illuminator.
III. Results
A. Clinical and postmortem examination
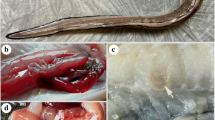
The examined fish showed no clinical signs. Most of Anisakid larvae were found attached to the visceral organs specially stomach and liver surfaces of the examined fish, also in the mesentery or free in the body cavity. Sometimes larvae were seen either free or encapsulated in the musculature. The infested liver showed paleness with hemorrhagic area around the encapsulated larvae “Plate. 1”.
B. Description of the detected Anisakid larvae
Microscopic examination revealed larval type consistent with Anisakis simplex, Pseudoterranova decipiens, Hysterothylacium spp and Contracaecum spp.” Plate. 2”
Anisakis simplex, (Rudolphi 1809): Description based on 30 third larval stages, it is 5.2-20 mm long X 0.25 mm wide. There is a boring tooth at the anterior end and excretory pore near it. The esophagus has an anterior muscular part ( 2-2.5 mm long) and a posterior ventriculus (0.5- 1.1 mm long). Both the intetstinal cecum and the ventricular appendix are absent. The tail is short 0.1 -0.22mm with a mucron at its tip.
Pseuoterranova decipiens (Krabbe,1878): Description based on 30 third larval stages, it is 25.2-28.5 mm long X, 0.74-0.82 mm wide. There is a boring tooth below the oral aperture and the excretory pore below the oral tooth.The esophagus is slender 3.5 mm long, ventriculus is elongate (0.9-1.1mm long), the ventricular appendix is absent and the intestinal cecum is 0.4-0.7 mm in length. The tail is (0.54mm long) and tipped with a mucron.
Hyserothylacium spp. (Ward and Magath, 1917): Description based on 15 third larva stage, it is 11.33- 12.4mm long X 0.14 -0.16mm wide. There is a boring tooth at the anterior end. The excretory pore is below the nerve ring. The length of the esophagus is 1.7-2.2 mm, the ventriculus is spherical in shape with a length of 0.1-0.15 mm. The intestinal cecum is short anteriorly projected (0.34-0.5 mm long) and the ventricular appendix is long (2.9- 3.8 mm). The tail is 0.7mm long and without a mucron.
Contracaecum spp. (Raillet and Henry, 1912): Descripion based on 10 third larval stages, it is 12.92-23.85 mm long X 0.52-0.82 mm wide. There is no anterior boring tooth. The excretory pore is near oral aperture. The length of the esophagus is 1.53-2.28 mm. The ventriculus is short subglobular (0.08-0.29 mm), ventricular appendix (0.5-1.3 mm long) is much shorter than intestinal cecum (1.08-1.89 mm long). The tail is conical (0.23mm long) and it has not a mucron.
C. Prevalence of Anisakid larvae among examined hakes
The seasonal prevalence of Anisakid larvae in both lizard head and European hake was the highest in spring followed by summer and winter and the lowest in autumn "Fig. 1; a and b”. The larvae were observed mostly within the body cavity and attached to the organs or in the musculature and its intensity ranged from 2-10 and 4-23 parasites per fish in the musculature and visceral organs (viscera and organs) of lizard head hakes respectively, while the range of Anisakid larvae intensity in European hakes was 1-4 and 3-25 parasites in the musculature and visceral organs respectively and the mean abundance of Anisakid larvae in the musculature and organs of lizard head was higher than that observed in European hake musculature and viscera “Table.1”.
D. Molecular identification of the Anisakid larvae
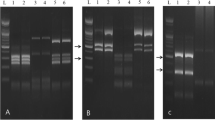
Data of RAPD-PCR analysis using the four arbitrary primers were shown in “Fig. 2” and Table. 3. All the analyzed RAPD primers were able to amplify the bulked DNA sample of Anisakid larvae. The range of RAPD-PCR band products was between three for ILO004 and five for ILO868, ILO524 and M13 primers. The molecular weights of the resultant bands ranged from 150 bp to 660 bp.
The PCR products of the bulked DNA sample generated by primer ILO868 revealed a maximum number of 5 bands with molecular weights ranging from 300 to 650 bp. The maximum number of generated clear bands by ILO524 were five with a molecular weight spectrum of 250 - 510 bp. The RAPD fingerprint of Anisakid using ILO004 had three distinct bands of 150, 390 and 550 bp. The RAPD identification of Anisakid larvae utilizing M13 was distinguished by five obvious PCR bands (300, 340, 440, 460 and 650 bp).
IV. Discussion
European (Merluccius merluccius) and Lizard head (Saurida undosquamis) hakes are considered as a common and commercially important fishes caught from Mediterranean Sea and gulf of Suez in Egypt respectively [19, 20] and distributed to all Egyptian governorates.
In the present study, the distribution of Anisakid larvae in the examined fish was highly related to the organs specially stomach and liver surfaces, mesentery, body cavity and musculatures. Most of larvae were attached to the viscera (encapsulated) or free in the body cavity of infested fish. These results were matched with those observed by [21, 22]. Also, the pathological findings observed in the organs were also nearly the same mentioned by the same authors [21, 22].
The morphological characteristics of Anisakis simplex, pseudoterrnova decipiens and contracaecum spp. larvae recorded in this study were in accordance with that described by [15, 23, 24, 25, 26, 27]. The morphological characters of Hysterothylacium larvae in the present study were similar to Hysterothylacium spp. type KE described by [27] in Caranx malabaricus, Lutjanus coccineus, Saurida undosquamis, Trichlurus lepturus and Argyrops spinifer in Kuwait. The detected species in the current study was different from that recorded by [10] in lizard head hakes in Sharkia province except for pseudoterrnova decipiens, this may be ascribed to the variation of the examined fish species. The infestation by Hysterothylacium and Contracaecum was less frequent than the other detected species, this may come in consistent with [28] who found that the Infestation with larvae of Anisakis occurs relatively frequently and [29, 30] who reported that the infestation with Hysterothylacium and Contracaecum is less common. It seems that the detected species of Contracaecum in the present study is C. multipapillatum. To our knowledge, both C. multipapillatum and Hysterothylacium spp. type KE were not previously recorded in this type of fish in Egypt, so, further genetic analysis is needed for a more accurate species identification.
Anisakid nematodes are widely distributed, and their prevalence among a variety of marine fish species had been previously identified [8, 9, 21, 31, 32]. In the current study the peak of the seasonal prevalence of Anisakid larvae was observed in spring and summer, nearly the same results was recorded by [22].This may be attributed to the temperature factor which enhance the life cycle of the parasite [33] and the abundance of natural food specially crustacean which is the main food taken by hake fishes in spring and summer [34] and play role in Anisakid life cycle. The intensity of Anisakid larvae was the highest in the viscera and organs in both examined spp. rather than in the musculature. Although a low number of the larvae was detected in the musculature, it is an indication that the larvae migrate to muscles after capture [35] and would increase the risk of human anisakidosis, furthermore there is a degree of cross reactivity between Anisakis allergens and other anisakids which render individuals sensitive to the allergens of other species [36]. This article presents a molecular genetic characterization using RAPD-PCR of the extracted Anisakid larvae populations. The four primers have been successfully used to determine the molecular genetic fingerprint of found Anisakids (Anisakis simplex, pseudoterranova decipiens, Hysterothylacium sp. type KE and contracaecum spp.) in respect to RAPD technique.
The limited number of the generated markers (3-5 PCR bands) provides for the first time a preliminary well-recognized genetic fingerprint of Anisakids infesting Egyptian coasts. Further studies are needed for assessment of genetic diversity, differentiation and phylogeny of Anisakids found in Egypt. Therefore, it is difficult to compare current study markers identifying fingerprint of one bulked Anisakid sample to other studies using more number of individual larval specimens. For instance, in a genetic diversity study by [37]; 143 RAPD-PCR bands were identified in 42 individual larval specimens of A. simplex infecting Spanish coasts using the same primers. Since the genetic fingerprint study can be done using one bulked DNA specimen.
V. Conclusion
The high prevalence of Anisakid larvae among examined lizard fish, which considered an important low priced meal for poor people in Egypt refers the possibility of human infection occurs after eating raw, under cooked or improperly processed fish which has adverse health effects in humans.In addition the allergens which is produced by either live or dead anisakis may lead to hypersensitivity to human, furthermore the cross reactivity between Anisakis allergens and other anisakids renders individuals sensitive to the allergens of other species. Moreover, this study provides well-identified genetic fingerprint for identification of anisakid larvae (Anisakis simplex, pseudoterranova decipiens, Hysterothylacium spp. and contracaecum spp.) and diagnosis of fish Anisakidosis. We argue further studying the genetic diversity and phylogenetic classification of Anisakids infecting Egyptian coasts.
Acknowledgement
We appreciate Prof. Dr. Adel A. Shaheen, Prof of Fish Diseases & Management for his helpful advices and useful information.
References
B. Szostakowska, P. Myjpk, M.Wyszynski, H. Pietkiewicz, J. Rokicki, “Prevalence of Anisakin nematodes in fish from southern Baltic sea,” Polish journal of microbiology, vol. 54, 2005, pp. 41–45.
T. L. Deardorff and M. L. Kent, “prevalence of larval Anisakis simplex in pen- reared and wild- caught salmon (Salmonidae) from Puget sound Washington,” JWD., vol. 25 (3), 1989, pp. 916–919.
A. Eguia, J.M. Aguirre, M.A. Echevarria, R. Martinez- Conde, and J. Pontón,“Gingivostomatitis after eating fish parasitized by Anisakis simplex, A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol,” Endod., vol. 96, 2003, pp. 437–440.
M. Montalto, L. Miele, A. Marcheggiano, L.Santoro, V. Curigliano, M. Vastola, and G. Gasbarrini, “Anisakis infestation: A case of acute .abdomen mimicking Crohn's disease and eosinophilic gastroenteritis,”. Digestive and Liver Disease, vol. 37, 2005, 62–64.
E.A. El-Daly, O.H. Amer, and T.I. Zaher, “Prevalence of Anisakid nematodes among marketed smoked and frozen marine fishes at Sharkia Governorate with special reference to their public health importance,” Z. U. M. J. Special Issue, vol. 11, 2004, pp. 647–655
M.T. Audicana, and M.W. Kennedy, “Anisakis simplex: From obscure infectious worm to inducer of immune hypersensitivity,”CMR., vol. 21, 2008, pp. 360–379.
R.C. Anderson“Nematode Parasites of Vertebrates Their Development and Transmission,” 2nd ed., CABI publisher, 2000, pp.
Z. Abd Al-Aal, O.H. Amer, A.A. El-Ashram, and W.T. El-Ekiaby,” Light and electron microscopic studies on some larvae in marine fishes,”. Zag. Vet. J., vol. 36, 2008, pp. 110–118
B.A. Ahmed, E.A. Desoky, O.H. Amer, and A.M. AbdEl-Ghany, “Larval anisakids (Nematoda: Ascaridoidea) in lizardfish (Saurida undosquamis) at Sharkia province Egypt,” Zag. Vet. J., vol. 38, 2010, pp. 168–175.
M.S.M. Nada and A. M. Amany, “Anisakid nematodes in marine fishes” Journal of American Science, vol. 7 (9), 2011, pp. 1000–1005.
S. Mattiucci, G.Nascetti, M. Dailey, S.C.Webb, R.Cianchi and L. Bullini, (2005) “Evidence for a new species of Anisakis Dujardin, 1845: morphological description and genetic relationships between congeners (Nematoda: Anisakidae),” Syst. Parasitol., vol. 61, 2005, pp. 157–171.
S. Mattiucci and G.Nascetti “ Genetic diversity and infection levels of anisakid nematodes parasitic in fish and marine mammals from Boreal and Austral hemispheres,” Vet Parasitol., vol. 148, 2007, pp. 43–57.
Z. Lucky, “Methods for the diagnosis of fishdiseases,” Amerind publishing Co. New Delhi, India Kruse, 1977.
F. Moravec, “Parasitic Nematodes of freshwater fishes of Europe,” Kluwer Academic publishers Dordrecht, Netherland, VI, 1994, pp. 470.
H. Shih,” Parasitic helminth fauna of the cutlass fish, Trichiurus lepturus L., and the differentiation of four anisakid nematode third-stage larvae by nuclear ribosomal DNA sequences,” Parasit. Res, vol. 93, 2004, pp. 188–195.
H. Shih, C. Ku, and C. Wang, “Anisakis simplex (Nematoda: Anisakidae) third-stage larval infections of marine cage cultured cobia, Rachycentron canadu in Taiwan,” Vet Parasitol., vol. 171(3-4), 2010, pp. 277–85
L. Margolis, G. W. Esch, J. C. Holmes, A. M. Kuris and G. A. Schad “The use of ecological terms in parasi-tology (Report of the American Society of Parasitologists),”;J Parasitol., vol. 6, 1982, pp. 131–133.
S. Gupta, and S. Preet, “ Protocol optimization for genomic DNA extraction and RAPD-PCR in mosquito larvae (Diptera: Culicidae),” Annals of Biological Research, vol. 3 (3), 2012, pp. 1553–1561.
M.A. Al-Absawy (2010) “The reproductive biology and the histological and ultrastructural characteristics in ovaries of the female gadidae fish Merluccius merluccius from the Egyptian Mediterranean water,” African Journal of Biotechnology, vol. 9, 2010, pp. 2544–2559.
A. M. Amin, M.M. EL-Halfawy and A.M Ramadan “Management and Reproduction of the Male Brushtooth Lizardfish Saurida undosquamis (Richardson) front Gulf of Suez, Egypt,” J. AquaL Biol & Fish, vol. ll.(4), 2007, pp. 149–162
I. A. M. Eissa, I.S. Gehan and A.S. Nashwa “Diseases caused by larval nematodes among some fishes in red sea,” SCVMJ., vol. XV (1), 2010, pp. 1–13.
M. A. Hassan, M.H. Abd El-Mohsen, and O.A.M. Hussien, “Some studies on Anisakidae larvae in some marine fish species,” Researcher, vol. 5(12), 2013, pp. 172–180
M. Koinari, S. Karl, A. Elliot, U. Ryan, A.J Lymbery, “Identification of Anisakis species (Nematoda: Anisakidae) in marine fish hosts from Papua New Guinea,” Vet Parasitol., vol. 193, 2013, pp. 126–133.
J. S. Hernández-Orts, F. J.Aznar, I. Blasco-Co, N.A. Garica, M. Víllora-Montero, E.A. Crespo, J.A. Raga and F.E. Montero, “Description, microhabitat selection and infection patterns of sealworm larvae (Pseudoterranova decipiens species complex, nematoda: ascaridoidea) in fishes from Patagonia, Argentina,” Parasite & vector, vol. 6 (252), 2013, pp. 215.
J. N Borges, L. F.G.Cunha, H. L. C Santos, C. Monteiro- Neto, C.P. Santos,” Morphological and Molecular Diagnosis of Anisakid Nematode Larvae from Cutlass fish (Trichiurus lepturus) off the Coast of Rio de Janeiro, Brazil,” Plos ONE, vol. 7, 2012, pp. 1–17.
J. O.Verbel, K. Caballero-Gallardo and B. Arroyo-Salgado, “Nematode infection in fish from Cartagena Bay, North of Colombia,” Vet Parasitol., vol. 177, 2011, pp. 119–126.
O. Sey and A.J. Petter, “Incidence of ascaridoid larvae in Kuwaiti food fishes”. Southeast Asian J Trop Med Public Health, vol. 28, 1997, pp. 168–72.
N. Kagei, H. Orikasa, E. Hori, A. Sannomiya, Y Yasumura, “ A case of hepatic anisakiasis with a literal surveyfor extra-intestinal anisakiasis,”. Jpn J Parasitol., vol. 44, 1995, pp. 346–351
K. Im, H. Shin, B. Kim and S.Moon, “Gastric anisakiasis cases in Cheju-do, Korea Republic,”. Korean J Parasitol., vol. 33, 1995, pp. 179–186
K. Yagi, K Nagasawa, H. Ishikura, A. Nakagawa, N. Sato, K. Kikuchi and H. Ishikura, “Female worm Hysterothylacium aduncum excreted from human: a case report,” Jpn J Parasitol., vol. 45, 1996, pp. 12–23.
J.L. Luque, and R..Poulin , 2004. “Use of fish as intermediate hosts by helminth parasites: A comparative analysis,” Acta Parasit., vol. 49, 2004, pp. 353–361
C. Cruz, Barbosa, C. and A. Saraiva, “Distribution of larval anisakids in blue whiting offPortuguese fish market,” Helminthologia, vol. 44, 2007, pp. 21–24.
I. A. Eissa, M., “Parasitic fish diseases in Egypt” Dar El-Nahda El-Arabia, Publishing, cairo, Egypt, vol. 32, 2002, pp. 149–160.
M. Ben-Yam, and T. Glaser, “The invasion of sarudia undosquamis (Richarson) into Levant basin an example of biological effect of inter-oceanic canals,” Fisheries pulletin, vol. 27(2), 1974, pp. 359–373.
M.V. Herreras, F.J Aznar, J.A. Balbuena and J.A. Raga, “Anisakid Larvae in the Musculature of the Argentinean Hake, Merluccius hubbsi. Journal of Food Protection, vol.63 (8), 2000, pp. 1141–1143.
J.L Maldonado, L.M.Hita, V.D. Saez, I.M.Almendros, A.V. Lopez, M.C. Bueno, “ Cross-reactivity between antigens of Anisakis simplex s.l. and other ascarid nematodes.” Parasite-Journal De La Societe Francaise De Parasitologie, vol. 11, 2004, pp. 219–223.
J. Martin-Sanchez, M.E.Artacho-Reinoso , M. D´ıaz-Gavilan and A.Valero-Lopez “Structure of Anisakis simplex s.l. populations in a region sympatric for A. pegreffii and A. simplex s.s. Absence of reproductive isolation between both species,” Molecular & Biochemical Parasitology, vol. 141, 2005,pp. 155–162.
Author information
Authors and Affiliations
Corresponding authors
Additional information
a Dept. of Fish Diseases & Management; b Dept. of Parasitology, c Dept. of animal wealth development and d Dept. of Food Hygeine. Faculty of Veterinary Medicine, Benha University, Egypt. www.bu.edu.eg
* Correspondence to: Reham S. Elmadawy1 , Amel M. El-Asely2 1E. mail: rehamelmadawy@hotmail.com, reham.almaadawy@fvtm.bu.edu.eg 2E-mail: amlvet@yahoo.com, amel.alaasly@fvtm.bu.edu.eg
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited..
Author’s Profile

Amel Mohamed ElAsely

Reham Sami ElMadawy
E-mail:rehamelmadawy@hotmail.com,
reham.almaadawy@fvtm.bu.edu.eg
Marwa Abdelkawy Eltanany, lecturer of animal wealth development, faculty of Veterinary Medicine, Benha University, 13736,Qalyobia Toukh, Moshtohor, +20 132473264, Fax: +20 132460640
Gehan Said Ahmed Afify, assistant prof, Department of food hygiene , faculty of Veterinary Medicine, Benha University, 13736,Qalyobia Toukh, Moshtohor, +20 132473264, Fax: +20 132460640
Appendix
Appendix
Figure A -M Larval stages of anisakid larvae. Anisakis Simplex. Fig. A. Anterior end. Fig. B. Ventricular region. Fig. C. Posterior end, Pseudoterrova decipiens. Fig. D. anterior end. Fig. E. Ventricular region. Fig. F. Posterior end. Hysterothylacium spp.. Fig.G. Anterior end. Fig. H. Ventricular region . Fig. I and J. Posterior end. Contracaecum spp. Fig. K. Anterior end. Fig.L. Ventricular region. Fig. M. Posterior end. Scale bar 0.5mm. Abbreviation: AT: anterior boring tooth, EP: excretory pore, V: ventriculus , M: mucron , OS: oesphagus, VA: Ventricular appendix, I: intestine, IC: Intestinal cecum, NR: nerve ring, AO: anal opening.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Asely, A., El Madawy, R., El Tanany, M. et al. Prevalence and Molecular Characterization of Anisakidosis in both European (Merluccius merluccius) and Lizard Head (Saurida undosquamis) Hakes. GSTF J Vet Sci 1, 1 (2015). https://doi.org/10.7603/s40871-015-0001-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40871-015-0001-3