Abstract
Background
Microscopic evaluation of parasite clearance is the gold standard in antimalarial drug efficacy trials. However, the presence of sub-microscopic residual parasitemia after artemisinin-based combination therapy (ACT) needs to be investigated.
Methods
One hundred and twenty (AL: n = 60, PA: n = 60) days 3 and 14 dried blood spots, negative by microscopy were analysed for residual parasitemia using nested PCR. Isolates with residual parasitemia on days 3 and 14 were further genotyped with their corresponding day-0 isolates using merozoite surface proteins msp-1, msp-2, and glurp genes for allelic similarity.
Results
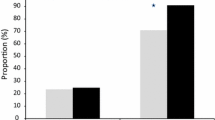
Persistent PCR-determined sub-microscopic residual parasitemia at day 3 post ACT treatment was 83.3 (AL) and 88.3% (PA), respectively (ρ = 0.600), while 63.6 and 36.4% (ρ = 0.066) isolates were parasitemic at day 14 for AL and PA, respectively. Microscopy-confirmed gametocytemia persisted from days 0 to 7 and from days 0 to 21 for AL and PA. When the alleles of day 3 versus day 0 were compared according to base pair sizes, 59% of parasites shared identical alleles for glurp, 36% each for 3D7 and FC27, while K1 was 77%, RO33 64%, and MAD20 23%, respectively. Similarly, day 14 versus day 0 was 36% (glurp), 64% (3D7), and 32% (FC27), while 73% (K1), 77% (RO33), and 41% (MAD20), respectively.
Conclusion
The occurrence of residual parasitemia on days 3 and 14 following AL or PA treatment may be attributable to the presence of either viable asexual, gametocytes, or dead parasite DNAs, which requires further investigation.


Similar content being viewed by others
Data availability
Available on reasonable request.
Materials availability
Available on reasonable request.
Code availability
Not applicable.
References
Amambua-Ngwa A, Okebe J, Mbye H, Ceesay S, El-Fatouri F, Joof F, Nyang H, Janha R, Affara M, Ahmad A, Kolly O, Nwakanma D, D'Alessandro U (2017) Sustained ex vivosusceptibility of Plasmodium falciparum toartemisinin derivatives but increasing toleranceto artemisinin combination therapy partnerquinolines in The Gambia. Antimicrob Agents Chemother 61:e00759-17. https://doi.org/10.1128/AAC.00759-17
Balikagala B, Fukuda N, Ikeda M, Katuro OT, Tachibana S-I, Yamauchi M, Opio W, Emoto S, Anywar DA, Kimura E et al (2021) Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 385:1163–1171. https://doi.org/10.1056/NEJMoa2101746
Bergmann C, van Loon W, Habarugira F, Tacoli C, Jäger JC, Savelsberg D, Nshimiyimana F, Rwamugema E, Mbarushimana D, Ndoli J et al (2021) Increase in Kelch 13 polymorphisms in Plasmodium falciparum, Southern Rwanda. Emerg Infect Dis 27:294. https://doi.org/10.3201/eid2701.203527
Beshir KB, Sutherland CJ, Sawa P, Drakeley CJ, Okell L, Mweresa CK, Omar SA, Shekalaghe SA, Kaur H, Ndaro A et al (2013) Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis 208:2017–2024. https://doi.org/10.1093/infdis/jit431
Byakika-Kibwika P, Nyakato P, Lamorde M, Kiragga AN (2018) Assessment of parasite clearance following treatment of severe malaria with intravenous artesunate in Ugandan children enrolled in a randomized controlled clinical trial. Malar J 17:400. https://doi.org/10.1186/s12936-018-2552-6
Carlsson AM, Ngasala BE, Dahlström S, Membi C, Veiga IM, Rombo L, Abdulla S, Premji Z, Gil JP, Björkman A et al (2011) Plasmodium falciparum population dynamics during the early phase of anti-malarial drug treatment in Tanzanian children with acute uncomplicated malaria. Malar J 10:380. https://doi.org/10.1186/1475-2875-10-380
Chang H-H, Meibalan E, Zelin J, Daniels R, Eziefula AC, Meyer EC, Tadesse F, Grignard L, Joice RC, Drakeley C et al (2016) Persistence of Plasmodium falciparum parasitemia after artemisinin combination therapy: evidence from a randomized trial in Uganda. Sci Rep 6:26330. https://doi.org/10.1038/srep26330
Dhorda M, Ba EH, Kevin Baird J, Barnwell J, Bell D, Carter JY, Dondorp A, Ekawati L, Gatton M, González I et al (2020) Towards harmonization of microscopy methods for malaria clinical research studies. Malar J 19:324. https://doi.org/10.1186/s12936-020-03352-z
Froeschl G, Saathoff E, Kroidl I, Berens-Riha N, Clowes P, Maboko L, Assisya W, Mwalongo W, Gerhardt M, Ntinginya EN et al (2018) Reduction of malaria prevalence after introduction of artemisinin-combination-therapy in Mbeya Region, Tanzania: results from a cohort study with 6773 participants. Malar J 17:245. https://doi.org/10.1186/s12936-018-2389-z
Funwei RI, Thomas BN, Falade CO, Ojurongbe O (2018) Extensive diversity in the allelic frequency of Plasmodium falciparum merozoite surface proteins and glutamate-rich protein in rural and urban settings of southwestern Nigeria. Malar J 17:1. https://doi.org/10.1186/s12936-017-2149-5
Garner P, Graves PM (2005) The benefits of artemisinin combination therapy for malaria extend beyond the individual patient. PLOS Med 2(4):e105. https://doi.org/10.1371/journal.pmed.0020105
Getnet G, Fola AA, Alemu A, Getie S, Fuehrer H-P, Noedl H (2015) Therapeutic efficacy of artemether–lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Enfranze, north-west Ethiopia. Malar J 14:258. https://doi.org/10.1186/s12936-015-0775-3
Hastings IM, Kay K, Hodel EM (2015) How robust are malaria parasite clearance rates as indicators of drug effectiveness and resistance? Antimicrob. Agents Chemother 59:6428–6436. https://doi.org/10.1128/AAC.00481-15
Imwong M, Suwannasin K, Kunasol C, Sutawong K, Mayxay M, Rekol H, Smithuis FM, Hlaing TM, Tun KM, van der Pluijm RW et al (2017) The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis 17:491–497. https://doi.org/10.1016/S1473-3099(17)30048-8
Jarra W, Snounou G (1998) Only viable parasites are detected by PCR following clearance of rodent malarial infections by drug treatment or immune responses. Infect Immun 66:3783–3787
Kiaco K, Teixeira J, Machado M, do Rosário V, Lopes D, (2015) Evaluation of artemether-lumefantrine efficacy in the treatment of uncomplicated malaria and its association with pfmdr1, pfatpase6 and K13-propeller polymorphisms in Luanda. Angola Malar J 14:504. https://doi.org/10.1186/s12936-015-1018-3
Lopera-Mesa TM, Doumbia S, Chiang S, Zeituni AE, Konate DS, Doumbouya M, Keita AS, Stepniewska K, Traore K, Diakite SAS et al (2013) Plasmodium falciparum clearance rates in response to artesunate in Malian children with malaria: effect of acquired immunity. J Infect Dis 207:1655–1663. https://doi.org/10.1093/infdis/jit082
Lubis IND, Wijaya H, Lubis M, Lubis CP, Beshir KB, Staedke SG, Sutherland CJ (2020) Recurrence of Plasmodium malariae and P. falciparum following treatment of uncomplicated malaria in North Sumatera with dihydroartemisinin-piperaquine or artemether-lumefantrine. Open Forum Infect Dis 7:ofaa116. https://doi.org/10.1093/ofid/ofaa116
Mhamilawa LE, Ngasala B, Morris U, Kitabi EN, Barnes R, Soe AP, Mmbando BP, Björkman A, Mårtensson A (2020) Parasite clearance, cure rate, post-treatment prophylaxis and safety of standard 3-day versus an extended 6-day treatment of artemether–lumefantrine and a single low-dose primaquine for uncomplicated Plasmodium falciparum malaria in Bagamoyo district, Tanzania: a randomized controlled trial. Malar J 19:216. https://doi.org/10.1186/s12936-020-03287-5
Mwaiswelo R, Ngasala B (2020) Evaluation of residual submicroscopic Plasmodium falciparum parasites 3 days after initiation of treatment with artemisinin-based combination therapy. Malar J 19:162. https://doi.org/10.1186/s12936-020-03235-3
Mwaiswelo R, Ngasala BE, Jovel I, Gosling R, Premji Z, Poirot E, Mmbando BP, Björkman A, Mårtensson A (2016) Safety of a single low-dose of primaquine in addition to standard artemether-lumefantrine regimen for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania. Malar J 15:316. https://doi.org/10.1186/s12936-016-1341-3
Mwaiswelo R, Ngasala B, Jovel I, Xu W, Larsson E, Malmberg M, Gil JP, Premji Z, Mmbando BP, Mårtensson A (2019) Prevalence of and risk factors associated with polymerase chain reaction-determined plasmodium falciparum positivity on day 3 after initiation of artemether–lumefantrine treatment for uncomplicated malaria in Bagamoyo District, Tanzania. Am J Trop Med Hyg 100:1179–1186. https://doi.org/10.4269/ajtmh.18-0729
Ohrt C, Purnomo P, Sutamihardja MA, Tang D, Kain KC (2002) Impact of microscopy error on estimates of protective efficacy in malaria-prevention trials. J Infect Dis 186:540–546. https://doi.org/10.1086/341938
Phyo AP, Nosten F (2018) The artemisinin resistance in Southeast Asia: an imminent global threat to malaria elimination. Malar Elimin - Leap Forw. https://doi.org/10.5772/intechopen.76519
Raman J, Kagoro FM, Mabuza A, Malatje G, Reid A, Frean J, Barnes KI (2019) Absence of kelch13 artemisinin resistance markers but strong selection for lumefantrine-tolerance molecular markers following 18 years of artemisinin-based combination therapy use in Mpumalanga Province, South Africa (2001–2018). Malar J 18:280. https://doi.org/10.1186/s12936-019-2911-y
Roth JM, Sawa P, Omweri G, Makio N, Osoti V, de Jong MD, Schallig HDFH, Mens PF (2018) Molecular detection of residual parasitemia after pyronaridine–artesunate or artemether–lumefantrine treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children. Am J Trop Med Hyg 99:970–977. https://doi.org/10.4269/ajtmh.18-0233
Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN (1993) High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61:315–320
Sowunmi A, Ntadom G, Akano K, Ibironke FO, Ayede AI, Agomo C, Folarin OA, Gbotosho GO, Happi C, Oguche S et al (2019) Declining responsiveness of childhood Plasmodium falciparum infections to artemisinin-based combination treatments ten years following deployment as first-line antimalarials in Nigeria. Infect Dis Poverty 8:69. https://doi.org/10.1186/s40249-019-0577-x
Sutherland CJ, Henrici RC, Artavanis-Tsakonas K (2020) Artemisinin susceptibility in the malaria parasite Plasmodium falciparum: propellers, adaptor proteins and the need for cellular healing. FEMS Microbiol Rev. https://doi.org/10.1093/femsre/fuaa056
Tadele G, Jaiteh FK, Oboh M, Oriero E, Dugassa S, Amambua-Ngwa A, Golassa L (2022) Persistence of residual submicroscopic P. falciparum parasitemia following treatment of artemether-lumefantrine in Ethio-Sudan Border, Western Ethiopia. Antimicrob Agents Chemother 0:e00002-22. https://doi.org/10.1128/aac.00002-22
Thriemer K, Hong NV, Rosanas-Urgell A, Phuc BQ, Ha DM, Pockele E, Guetens P, Van NV, Duong TT, Amambua-Ngwa A et al (2014) Delayed parasite clearance after treatment with dihydroartemisinin-piperaquine in Plasmodium falciparum malaria patients in Central Vietnam. Antimicrob Agents Chemother 58:7049–7055. https://doi.org/10.1128/AAC.02746-14
Tjitra E, Suprianto S, Anstey NM (2002) Higher gametocyte prevalence following failure of treatment of Plasmodium falciparum malaria with sulfadoxine-pyrimethamine and the combination of chloroquine plus sulfadoxine-pyrimethamine: implications for progression of anti-folate resistance. Trans R Soc Trop Med Hyg 96:434–437. https://doi.org/10.1016/S0035-9203(02)90385-8
Vafa Homann M, Emami SN, Yman V, Stenström C, Sondén K, Ramström H, Karlsson M, Asghar M, Färnert A (2017) Detection of malaria parasites after treatment in travelers: a 12-months longitudinal study and statistical modelling analysis. EBioMedicine 25:66–72. https://doi.org/10.1016/j.ebiom.2017.10.003
Vinayak S, Alam MT, Mixson-Hayden T, McCollum AM, Sem R, Shah NK, Lim P, Muth S, Rogers WO, Fandeur T et al (2010) Origin and evolution of sulfadoxine resistant Plasmodium falciparum. PLOS Pathog 6:e1000830. https://doi.org/10.1371/journal.ppat.1000830
White NJ (2017) Malaria parasite clearance. Malar J 16:88. https://doi.org/10.1186/s12936-017-1731-1
Zwang J, Dorsey G, Mårtensson A, d’Alessandro U, Ndiaye J-L, Karema C, Djimde A, Brasseur P, Sirima SB, Olliaro P (2014) Plasmodium falciparum clearance in clinical studies of artesunate-amodiaquine and comparator treatments in sub-Saharan Africa, 1999–2009. Malar J 13:114. https://doi.org/10.1186/1475-2875-13-114
Acknowledgements
We appreciate all study participants and their parents/guardians. We also thank our research and clinical staff for their commitment during and after field work.
Funding
The clinical aspect of the study was funded by Shin Poong Pharmaceutical Company, Ltd., Ansan, South Korea.
Author information
Authors and Affiliations
Contributions
Roland I. Funwei and Catherine O. Falade conceived, designed, and supervised the study. Olusola Ojorungbe and Oladapo Walker co-supervised and edited the scientific content in the manuscript. Roland I. Funwei, Wasiu A. Hammed, and Gabriel N. Uyaiabasi performed the laboratory procedures and analyzed the data. Roland I. Funwei drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Ethical approval was obtained from the University of Ibadan/University College Hospital Institutional Review Board (UI/UCH/IRB).
Consent to participate
Written informed consent was obtained from all parents/guardians before enrollment into the study. Participants were free to withdraw if they felt otherwise to continue with the study.
Consent for publication
Details of participants were not disclosed in the manuscript. Therefore, it can be published.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Julia Walochnik
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Funwei, R.I., Uyaiabasi, G.N., Hammed, W.A. et al. High prevalence of persistent residual parasitemia on days 3 and 14 after artemether–lumefantrine or pyronaridine–artesunate treatment of uncomplicated Plasmodium falciparum malaria in Nigeria. Parasitol Res 122, 519–526 (2023). https://doi.org/10.1007/s00436-022-07753-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07753-8