Abstract
Encephalitozoon cuniculi is a microsporidian parasite mostly associated with its natural host, the rabbit (Oryctolagus cuniculus). However, other animals can be infected, like other mammals, birds, and even humans. Although it usually causes subclinical infection, it can also lead to encephalitozoonosis, a clinical disease characterized by neurological, ocular, and/or renal signs that can be even fatal, especially in immunocompromised individuals. Therefore, this multidisciplinary review contributes with updated information about the E. cuniculi, deepening in its molecular and genetic characterization, its mechanisms of infection and transmission, and its prevalence among different species and geographic locations, in a One Health perspective. Recent information about the diagnostic and therapeutic approach in the main host species and the prophylaxis and infection control measures currently suggested are also discussed.
Similar content being viewed by others
Introduction
Encephalitozoon cuniculi (E. cuniculi) is an obligate intracellular and spore-forming microsporidian parasite that can infect many species of mammals, such as lagomorphs, rodents, dogs, cats, horses, ruminants, wild and exotic carnivores, nonhuman primates and even humans, but also some bird species (Deplazes et al. 2000). This pathogen is usually associated with subclinical chronic infections, but it can also lead to a serious clinical illness named encephalitozoonosis and even death (Wasson and Peper 2000; Künzel and Fisher 2018).
This literature review will address the impact of Encephalitozoon cuniculi as an opportunistic zoonotic agent in the One Health context, highlighting its worldwide presence among different species and reinforcing the importance of its proactive investigation, treatment, and prevention. In addition to humans, the main animal species covered in this article are rabbits, as the natural hosts of E. cuniculi, rodents as the frequent reservoirs of infectious diseases, and small companion animals, such as dogs and cats, due to their close connection with humans, with whom they share the same living environment. Even so, the presence of this microsporidial parasite in other species will also be briefly discussed, since its potential role cannot be overlooked.
Therefore, the main goal of this work is to contribute with updated and objective information about this zoonosis, promoting the awareness of health professionals, from human and veterinary medicine, for the importance of this often underdiagnosed disease that can lead to important public health issues, especially in immunocompromised individuals.
Characterization of Encephalitozoon cuniculi
Microsporidia were considered ancient and basic eukaryotic organisms due to their lack of typical mitochondria, Golgi apparatus, and peroxisomes and because they have structures closer to those found in prokaryotes, such as small ribosomes (Didier and Weiss 2006). In fact, a close phylogenetic relation to fungi has been supported by analyses of multiple gene sequences and due to the presence of fungal components, such as tubulins, chitin, and trehalose (Didier and Weiss 2006; Bohne et al. 2011). A determining feature for their ability to infect is the presence of the polar tube, a coiled filamentous hollow tube composed by proteins that is contained within their spores (Xu and Weiss 2005).
In 1995, Didier et al. (1995) described the existence of at least three E. cuniculi strains (I, II, and III), through the identification of a clear difference in the internal transcribed spacer (ITS) region of the ribosomal RNA gene. At this level, the sequence 5′-GTTT-3′ repeats 3 times in strain I, twice in strain II, and 4 times in strain type III (Didier et al. 1995). More recently, a novel type IV was identified in a human patient, exhibiting 5 repeats of 5′-GTTT-3′ in the ITS region (Talabani et al. 2010). The type of strain was eventually associated with the animal species in which it was isolated, so type I is considered the “rabbit strain”, type II is called the “mouse strain”, type III is designated the “dog strain”, and type IV is referred to as the “human strain” (Mathis et al. 2005; Talabani et al. 2010). However, although each strain has a preferential host species, in practice, it is currently known that E. cuniculi has a low host specificity. For example, genotype I was also detected in horses (Wagnerová et al. 2012) and gorillas and chimpanzees (Sak et al. 2011b), genotype II in blue foxes (Mathis et al. 1996) and cats (Benz et al. 2011), genotype III in wild small rodents (Hofmannová et al. 2014; Perec-Matysiak et al. 2019) and pigs (Reetz et al. 2009), and genotype IV in dogs (Nell et al. 2015). In addition, in humans, apart from strain type IV, the other three strains (I, II, and III) were also diagnosed, proving its zoonotic potential (Sak et al. 2011a).
New alternative tools have been recently suggested for the genotyping of E. cuniculi, based on the length polymorphism and the sequence diversities of the polar tube protein (PTP) and the spore wall protein I (SWP-1) genes (Xiao et al. 2001). These novel polymerase chain reaction (PCR) techniques could be a more easily accessible option to most laboratories, and their results (particularly the sequence analysis of the PTP gene) showed agreement with the previous three genotypes reported by the ITS sequence analysis (Xiao et al. 2001).
Host cell infection and immune system response
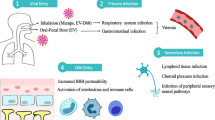
Once inside the host’s body, the spore is subject to a set of stimuli that result in an increase in osmotic pressure that leads to the eversion and the mechanic extrusion of the polar tube and to the consequent host cell invasion, through perforation or, as currently more accepted, through a channel-like invagination of the plasma membrane of the host cell (Rönnebäumer et al. 2008; Bohne et al. 2011) (Fig. 1). This first set of phenomena is known as the germination stage (Xu and Weiss 2005; Bohne et al. 2011). Thus, the infective sporoplasm is transferred into the host cell, forming at the same time a parasitophorous vacuole (PV) around it, where it matures into simple structures called meronts, which in turn undergo a process of asexual reproduction, named merogony and described as an intense replication process through binary or multiple fission (Fasshauer et al. 2005; Bohne et al. 2011). After that, the meronts differentiate inside the PV into more morphologically and structurally complex structures, through the acquisition of the resistant spore wall and the characteristic polar tube inside, leading to the formation of sporons and then mature spores, completing the third stage designated sporogony (Bohne et al. 2011). With the continued formation and accumulation of spores within the intracellular space, the host cell eventually ruptures and releases spores into extracellular spaces, spreading the infection locally, through direct extension to the surrounding cells, and at a distance through invasion of the vascular system (Wasson and Peper 2000). Thus, the main target organs of this microsporidian parasite are those with high blood flow, leading to the formation of granulomatous lesions, as result of exuberant inflammatory infiltrates, mainly in the kidneys, eyes, and brain, but also in the liver, lungs, and heart (Latney et al. 2014).
As an alternative to the process of cell invasion described, dependent on extrusion of the polar tube, microsporidia can infect host cells at the moment they undergo phagocytosis (Fasshauer et al. 2005; Dalboni et al. 2021). After that, the two stages of intracellular proliferation (merogony) and differentiation (sporogony) occur as mentioned before (Fasshauer et al. 2005). The nutrient uptake that is essential for all intracellular stages of proliferation, development, and differentiation is ensured through the pores present in the PV membrane that allow the entry of small molecules from the cytoplasm of the host cell (Rönnebäumer et al. 2008).
After infection, the host compromised cells induce the release of Th1 cytokines, such as interleukin 12 (IL-12) and interferon gamma (IFN-γ), establishing an immune response mediated by CD8 + T cells, which leads to the destruction of the infected cells, through the perforin pathway (Khan et al. 2001). Moreover, the proliferation of CD4 + cells is also described as part of the cell-mediated immunity (Sak et al. 2017; Jeklova et al. 2020) and, although with lesser protective effect, humoral immunity through the production of immunoglobulins against this pathogen is also present (Jeklova et al. 2020). In fact, it seems that higher antibody titers are related to the acute phase of infection since a more accentuated spore multiplication is expected at this stage (Levkutová et al. 2004; Wagnerová et al. 2013).
Despite this host immune response to control the infection, E. cuniculi can evade it in different ways. One of the main mechanisms is by taking advantage of the efferocytosis process, a natural phagocytic mechanism that occurs after cell death (mainly after apoptosis): the pathogen is able to multiply inside the macrophages and to modulate their activity, inducing the production of anti-inflammatory cytokines, such as IL-6 and IL-10, and consequently promoting a favorable environment for its dissemination (Dalboni et al. 2021). Moreover, the lack of host cell marker proteins in the PV membrane allows it to remain undetected inside the cells, avoiding the lysosomal digestion (Bohne et al. 2011).
Epidemiology and serological prevalence
Rabbits (Oryctolagus cuniculus) are considered the natural host of E. cuniculi, but a wide range of species can be affected, including humans, which makes it a pathogen with low host specificity, as mentioned above (Deplazes et al. 2000). In addition to not being a species-specific infection, there also seems to be no association with other characteristics of the animal. For example, E. cuniculi appears to be unrelated to the individual’s sex/gender, as described in rabbits (Keeble and Shaw 2006; Dipineto et al. 2008; Okewole 2008; Jeklova et al. 2010; Tee et al. 2011; Shin et al. 2014; Berger Baldotto et al. 2015), cats (Benz et al. 2011; Tsukada et al. 2016), humans (Abu-Akkada et al. 2015b), and horses (Wagnerová et al. 2012), and even unrelated to age (Keeble and Shaw 2006; Okewole 2008; Jeklova et al. 2010; Shin et al. 2014; Berger Baldotto et al. 2015). However, in terms of age, rabbits older than 4 months (Dipineto et al. 2008; Santaniello et al. 2009) and horses older than 3 years (Wagnerová et al. 2012) showed a significantly higher seropositivity in comparison with the respective younger counterparts. The reason given in the two studies carried out in rabbits to justify this discrepancy is that the young animals were tested between 1 and 2 months, when they are typically seronegative, as maternal antibodies have just stopped being present (Dipineto et al. 2008). Further studies are needed to investigate possible epidemiological associations.
Concerning its geographical distribution, E. cuniculi have a worldwide distribution, with cases reported over the five continents (Thomas et al. 1997; Okewole 2008; Lindsay et al. 2009; Pan et al. 2015; Maestrini et al. 2017).
The infection by this pathogen is mostly asymptomatic, so the serological detection of IgG antibodies against E. cuniculi has been performed in human and animal populations in order to assess the seroprevalence of this microsporidian in different species and geographic locations around the globe. Therefore, the estimated prevalence of E. cuniculi described through serological screening in the main species with zoonotic interest (rabbits, small companion animals, and humans) and in different geographic regions is systematized as shown in Table 1.
Potential transmission routes, environmental resistance, and risk factors
The most common transmission routes in mammals are through ingestion or inhalation of spores present in the environment that were previously excreted by infected patients via urine (mainly) or feces (Künzel et al. 2008; Latney et al. 2014) (Fig. 2). Moreover, it is assumed that microsporidia can be transmitted to humans through food, such as milk (Kváč et al. 2016), fermented meat products (Sak et al. 2019), and products from crops (Thurston-Enriquez et al. 2002).
Kváč et al. (2016) reported for the first time the presence of E. cuniculi in cow’s milk and showed that its spores remain infective even after a high-temperature short-time (HTST) pasteurization process (72 °C for 15 s or 85 °C for 5 s). Moreover, Sak et al. (2019) recently suggested that fermented pork products may represent an additional possible source of infection, considering the results of their study that show the presence of spores in pork meat samples, using a nested PCR protocol, and that the fermentation process is not effective for spore inactivation, under experimental conditions. Rabbit meat can also be a source of infection for humans, as in some countries this type of meat is often consumed (Wang et al. 2018). Finally, products from crops, which are often consumed raw, could also be sources of infection, as the presence of microsporidia spores in irrigation water has been reported (Thurston-Enriquez et al. 2002).
In addition to the horizontal transmission, vertical transmission has been also described, through the placenta, in different hosts species, such as rabbits (Baneux and Pognan 2003), dogs (Snowden et al. 2009), and mice (Kotková et al. 2018), and has been presumed in an infected snow leopard (Scurrell et al. 2015) and in emperor tamarins (Guscetti et al. 2003; Juan-Sallés et al. 2006).
E. cuniculi is present in the environment in the form of a spore, which is quite resistant due to its wall formed by an inner layer with chitin and an outer layer enriched in protein (Bohne et al. 2011). It is known that E. cuniculi spores have the ability to survive up to 6 weeks at 22 °C outside the host’s body (Künzel and Fisher 2018) and even longer in water environments, such as ditch water and river water, at the proper temperature (Li et al. 2003). Li et al. (2003) conducted an experimental study that evaluated the infectivity of culture-derived E. cuniculi spores stored in water, at various temperatures, and concluded that spores were still infective for 3 months, 2 months, 1 month, 3 weeks, and only 1 week at 10 °C, 15 °C, 20 °C, 25 °C, and 30 °C, respectively. Even so, E. cuniculi spores apparently lose infectivity more rapidly than other microsporidia species that infect mammals, such as Encephalitozoon hellem and Encephalitozoon intestinalis (Li et al. 2003).
Regarding risk factors and conditions that favor infection, the immunocompromised health status of the individual has been described as the main factor favoring infection by this opportunistic agent (Didier 2005), but other factors have been reported in animals and humans. For example, in animals, some housing and food conditions have been highlighted: the rearing on household farms, in contrast to commercial farms, due to the less hygienic-sanitary conditions normally found in the first places (Wang et al. 2018); the use of multi-animal communal cages due to the higher animal density and the consequent contact with urine and feces from other animals (Okewole 2008); the fruit-, vegetable-, and grain-based diets due to the potential contamination of these foods (Wang et al. 2018; Marková et al. 2019); the outdoor life as stray/feral animals instead of exclusive indoor life like pet animals (Addie et al. 2020); and keeping animals in stable systems rather than on pasture or in paddocks (Wagnerová et al. 2012). In humans, other risk factors have been suggested: working with animals and animal products (Halánová et al. 2003); handling animals and washing their cages due to the potential contact with the urine of infected individuals (Ozkan et al. 2011; Sak et al. 2011b; Carhan et al. 2015); living in poor sanitary conditions (Halánová et al. 2013); and outdoor activities in wildlife habitats, since wild animals are considered reservoirs of this disease (Murphy et al. 2007; Meredith et al. 2015).
Diagnosis: serological, molecular, and histopathological examinations
Serological testing
Serological testing has been used for the detection of antibodies against E. cuniculi in order to confirm/exclude the presence of infection in suspected animals or even to identify asymptomatic carrier individuals. Although most studies only search for IgG antibodies against E. cuniculi, the simultaneous detection with IgM has been described, which allows the differentiation between active (acute, reactivation, or reinfection) and chronic/latent infection (Igarashi et al. 2008; Jeklova et al. 2010): there is typically an increase in IgM titers from day 0 to day 35 post-exposure and an increase in IgG titers only at 2 and 3 weeks after exposure (Cox et al. 1979). This serological aspect is important because it influences the therapeutic decision: a positive titer of IgM antibodies in a suspected animal is sufficient to establish the diagnosis and initiate intensive treatment (Jeklova et al. 2010). On the other hand, a positive titer of IgG can indicate a potential current infection (with at least 3 weeks from exposure), a chronic infection, or a recovered previous infection (Cray and Rivas 2013; Latney et al. 2014). Therefore, a suspected individual with a negative titer allows the clinician to rule out the E. cuniculi as the causative agent of the disease, whereas if it has a positive titer, the clinical suspicion is reinforced, but it is still not possible to prove that the present clinical signs are caused by E. cuniculi (Csokai et al. 2009).
The serological techniques that have already been described for this detection are the enzyme-linked immunosorbent assay (ELISA), the indirect fluorescence antibody test (IFAT), the carbon immune assay (CIA), the quantitative western blot (qWB) analysis, and the direct agglutination test (DAT) (Boot et al. 2000; Jordan et al. 2006b; Desoubeaux et al. 2017).
As shown in Table 1, ELISA and IFAT are the most commonly used serological techniques, because they are easy to perform, allow quick results, and are currently well known and widely available (Boot et al. 2000; Csokai et al. 2009). A good agreement has been described between the two assays (Boot et al. 2000). From a study in which IFAT was the approach used, it was noticed that the IgG immunoglobulin response is triggered against the spore wall and not to the polar tube as previously thought (Talabani et al. 2010).
CIA has been used in seroprevalence studies mainly in rabbits (Dipineto et al. 2008; Santaniello et al. 2009; Ozkan et al. 2011; Tee et al. 2011; Lonardi et al. 2013; Hein et al. 2014; Lavazza et al. 2016), and it has been considered as an inexpensive and quick serological technique with a good correlation with ELISA (Dipineto et al. 2008) and IFAT (Boot et al. 2000; Hein et al. 2014) results. The main limitation remains in its later positive results compared to other methods, since it only has the ability to measure IgG, which means it only detects seropositivity when 2–3 weeks have elapsed from the onset of infection (Boot et al. 2000; Lavazza et al. 2016).
Quantitative western blot analysis allows a “one-step technical approach” for IgG- and IgM-antibody-specific detection (Desoubeaux et al. 2017) and has been applied in populations of cats (Künzel et al. 2014), rabbits (Desoubeaux et al. 2017), and humans (Deplazes et al. 1996), as a reliable serological alternative. However, in the routine practice, it is not often applied due to its labor-intensive and time-consuming features and its similar results with faster and easier techniques, such as IFAT, which end up being preferred (Künzel et al. 2014; Desoubeaux et al. 2017).
Lastly, DAT has been described for the detection of antibodies against E. cuniculi in mice, horses, and dogs, with a sensitivity and specificity that varies between 68.0 and 94.0% and between 89.0 and 98.0%, respectively, according to the examined species (Goodwin et al. 2006; Jordan et al. 2006b; Lindsay et al. 2009). The main advantages over the other serological assays are not needing specialized equipment, being faster and easier to perform, the lower risk of cross-reactivity, and the ability to be applied to a greater number of species as it does not require species-specific antibodies (Jordan et al. 2006b). Even so, in cats, DAT is not yet validated, and the study by Meredith et al. (2015) raised the possibility that it may not be appropriate in this species, as they have not detected any seropositive cats, within a population from UK, through this serological methodology. Thus, its application in other species is necessary to confirm its diagnostic value, which may increase its use in the future.
The detection of IgG antibodies against E. cuniculi spore wall antigen or E. cuniculi polar tube antigen has been carried out also in urine samples by an ELISA technique, but the uropositivity was only associated with strongly seroreactive rabbits, so further studies are needed to confirm the usefulness of this approach (Furuya et al. 2009).
Molecular testing
Another diagnostic method is the detection of E. cuniculi deoxyribonucleic acid (DNA) by polymerase chain reaction (PCR), by either conventional, nested, or real-time technique (Csokai et al. 2009; Zietek et al. 2014).
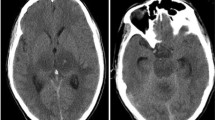
Nested or real-time PCR allows the detection of E. cuniculi DNA in organ and tissue samples, such as brain, kidney, liver, lung, spleen, and heart (Csokai et al. 2009; Leipig et al. 2013). The brain has been considered the most appropriate tissue for the detection of E. cuniculi DNA, since it is probably the organ with the highest concentration of spores in infected individuals (Csokai et al. 2009; Leipig et al. 2013). The main disadvantages of this technique are that it only allows a postmortem diagnosis, since the tissues are usually obtained at necropsy, and the fact that there may be false negative results, due to a low concentration or a non-uniform distribution of spores in the tissue samples examined (Csokai et al. 2009). However, in rabbits and cats, conventional PCR detection of E. cuniculi DNA from a sample of aqueous humor or liquefied lens has been considered as an excellent method for the antemortem diagnosis of patients with phacoclastic uveitis (Csokai et al. 2009; Künzel et al. 2014).
In addition to organs and tissues, nested or real-time PCR has been also carried out in animals and humans using samples from feces (Deplazes et al. 1996; Weber et al. 1997; Fournier et al. 2000; Lobo et al. 2003; Sak et al. 2011a; Sak et al. 2011b; Perec-Matysiak et al. 2019), urine (Deplazes et al. 1996; Fournier et al. 2000; Baneux and Pognan 2003; Csokai et al. 2009; Hein et al. 2014; Zietek et al. 2014; Abu-Akkada et al. 2015a; Boer et al. 2021), and cerebrospinal fluid (Csokai et al. 2009; Hein et al. 2014; Jeklova et al. 2020), allowing the diagnosis of the disease in clinical cases and the identification of infected animals excreting spores in epidemiological prevalence surveys. The PCR technique in urine (Baneux and Pognan 2003; Csokai et al. 2009) and in cerebrospinal fluid (Csokai et al. 2009; Jeklova et al. 2020) has been associated with low sensitivity, which can lead to false negative results. The currently accepted reason for the low sensitivity of this molecular screening method on urine samples is the intermittent excretion of spores, which decreases during the course of the infection (Cox et al. 1979). In fact, according to Cox et al. (1979), a significant amount of E. cuniculi spores was excreted in urine of acutely infected rabbits until day 63, but after that the excretion became intermittent and in smaller amounts, ceasing around 98 days post-infection. Besides that, in humans, PCR testing has also been effectively carried out in other body fluids, such as bronchoalveolar washings (Deplazes et al. 1996; Kicia et al. 2019), pleural fluid (Kicia et al. 2019), and sputum (Weber et al. 1997; Fournier et al. 2000; Talabani et al. 2010; Kicia et al. 2019).
Histological and immunological examination
Histopathology allows the identification of lesions compatible with infection by E. cuniculi, such as granulomatous interstitial nephritis and granulomatous encephalitis in association with macrophage and lymphocyte infiltration and fibrosis (Leipig et al. 2013; Rodríguez-Tovar et al. 2017). Yet, similar lesions have also been described in the heart, lungs, and liver of infected animals (Rebel-Bauder et al. 2011; Leipig et al. 2013). A high correlation between the intensity of the lesions and the levels of antibody titers has been described (Csokai et al. 2009).
The histological examination also enables the identification and evaluation of spores in paraffin-embedded tissues when samples are stained with Gram’s stain (Guscetti et al. 2003; Maestrini et al. 2017; Rodríguez-Tovar et al. 2017), Ziehl–Neelsen (ZN) (Csokai et al. 2009; Maestrini et al. 2017; Rodríguez-Tovar et al. 2017), acid-fast trichrome (AFT) (Csokai et al. 2009; Maestrini et al. 2017), calcofluor white stain (Rodríguez-Tovar et al. 2017), and modified trichrome staining (MTS) (Reetz et al. 2009; Rodríguez-Tovar et al. 2017). The preferred stains for detection of spores of E. cuniculi in tissues are MTS and Gram’s stain, by light microscopy, and calcofluor white stain, by ultraviolet light microscopy (Rodríguez-Tovar et al. 2017).
In addition, immunohistochemistry has been used mainly through monoclonal anti-E. cuniculi antibodies in tissue sections obtained from suspected animals (Mo and Drancourt 2004; Maestrini et al. 2017; Jeklova et al. 2020).
Other diagnostic tests
Transmission electron microscopy examination, often referred to as TEM, is another effective diagnostic approach to enhance the identification of the microsporidia in tissue samples and that even allows the differentiation of its stage (meront, sporont, and sporoblast) (Illanes et al. 1993; Guscetti et al. 2003; Juan-Sallés et al. 2006; Morsy et al. 2020).
Cerebral spinal fluid analysis has been also reported and usually shows a lymphomonocytic pleocytosis and a high protein level, laboratorial abnormalities common to other brain diseases, which result in little diagnostic value (Jass et al. 2008), so, given the inherent risk of the technique, this approach is no longer advised (Künzel and Fisher 2018).
Finally, cytological examination of aqueous humor or lens material (Benz et al. 2011) and urine staining with trichrome stain (Jass et al. 2008) were previously described, but the low sensitivity associated with both assessments limits their diagnostic value.
Infection in the main host species: clinical presentation and treatment
Rabbits
Most infections in rabbits are subclinical, with a large percentage of clinically healthy positive rabbits detected in serological screenings (Keeble and Shaw 2006; Csokai et al. 2009; Lonardi et al. 2013; Neumayerová et al. 2014; Maestrini et al. 2017). Even so, rabbits with clinical manifestation of infection can show three main presentations: neurological, renal, and ocular (Künzel and Fisher 2018).
The neurological condition is the most reported in this species, and the clinical signs are related to a central vestibular dysfunction, such as head tilt (Dipineto et al. 2008; Okewole 2008; Csokai et al. 2009; Ashmawy et al. 2011; Tee et al. 2011; Berger Baldotto et al. 2015; Lavazza et al. 2016), torticollis (Ozkan et al. 2011; Fukui et al. 2013; Zietek et al. 2014; Maestrini et al. 2017; Morsy et al. 2020), tremors (Dipineto et al. 2008; Jeklova et al. 2010), circling (Jeklova et al. 2010; Lavazza et al. 2016; Morsy et al. 2020), ataxia (Dipineto et al. 2008; Jeklova et al. 2010; Fukui et al. 2013; Lavazza et al. 2016; Morsy et al. 2020), nystagmus (Jeklova et al. 2010; Lavazza et al. 2016), seizures (Jeklova et al. 2010), and asthenia, paresis, or even paralysis of limbs (Dipineto et al. 2008; Okewole 2008; Ashmawy et al. 2011; Ozkan et al. 2011; Zietek et al. 2014; Berger Baldotto et al. 2015; Morsy et al. 2020). Urinary incontinence was also recorded (Okewole 2008; Zietek et al. 2014; Berger Baldotto et al. 2015). In turn, the ocular presentation is described as phacoclastic uveitis, characterized by severe inflammation of the uvea, the appearance of whitish intraocular lesions and even cataracts (Giordano et al. 2005; Dipineto et al. 2008; Csokai et al. 2009; Jeklova et al. 2010; Tee et al. 2011; Berger Baldotto et al. 2015; Lavazza et al. 2016; Morsy et al. 2020). The renal presentation is noted as chronic interstitial nephritis, associated with signs of kidney disease, such as polyuria/polydipsia (Dipineto et al. 2008; Jeklova et al. 2010; Morsy et al. 2020), dehydration (Morsy et al. 2020), anorexia (Morsy et al. 2020), and weight loss (Jeklova et al. 2010; Morsy et al. 2020). Rabbits can show only one presentation or even a combination of these conditions (Dipineto et al. 2008; Okewole 2008; Jeklova et al. 2010; Tee et al. 2011; Berger Baldotto et al. 2015; Lavazza et al. 2016), and in some cases, this clinical condition can also lead to death (Fukui et al. 2013; Maestrini et al. 2017).
Fenbendazole is the drug of choice for the treatment of E. cuniculi at a dose of 20 mg/kg per day for 28 consecutive days (Suter et al. 2001). This drug allows the elimination, or at least reduction, of spores present in the affected tissues, controlling the microsporidial infection (Suter et al. 2001). Alternatively, the use of albendazole and oxibendazole is occasionally reported (Giordano et al. 2005; Desoubeaux et al. 2017). Care should be taken in patients undergoing these drugs as benzimidazole toxicosis has been described in rabbits, mainly with albendazole and when doses higher than the standard reference range or for longer than recommended are given (Graham et al. 2014). In addition to the targeted treatment, supportive therapies are also described to control clinical signs and lesions secondary to the infection. For example, phacoemulsification or even enucleation has been required in rabbits with phacoclastic uveitis (Giordano et al. 2005; Csokai et al. 2009) and physiotherapy has been suggested for rabbits with central vestibular dysfunction (Künzel and Fisher 2018).
Rodents
Rodents have often been used to study E. cuniculi by allowing, in an experimental context, the evaluation of the parasite dissemination within the host’s body (Kotkova et al. 2013; Sak et al. 2017), the possibility of vertical transmission (Kotková et al. 2018), and the therapeutic response to drugs (Kotkova et al. 2013; Lallo et al. 2013; Sak et al. 2017, 2020).
Furthermore, it is known that wild rodents are capable of being infected by this agent, functioning as potential “reservoirs” and sources of transmission for predatory species, other domestic animals, and even humans, as described in epidemiological surveys (Hersteinsson et al. 1993; Meredith et al. 2015; Perec-Matysiak et al. 2019) and case reports (Hofmannová et al. 2014; Kitz et al. 2018). For example, the overall prevalence of E. cuniculi in wild rodents was 5.31% in the UK (Meredith et al. 2015) and 15% in Central Europe (Perec-Matysiak et al. 2019), according to epidemiological data obtained through serological and molecular methods, respectively.
Thus, infection by E. cuniculi has been reported in several species of small rodents, such as the house mouse (Mus musculus) (Hersteinsson et al. 1993), the striped field mouse (Apodemus agrarius) (Perec-Matysiak et al. 2019), the yellow-necked mouse (Apodemus flavicollis) (Perec-Matysiak et al. 2019), the long-tailed field mouse/wood mouse (Apodemus sylvaticus) (Hersteinsson et al. 1993; Meredith et al. 2015; Perec-Matysiak et al. 2019), the barbary striped grass mouse (Lemniscomys barbarus) (Kitz et al. 2018), the bank vole (Myodes glareolus) (Meredith et al. 2015; Perec-Matysiak et al. 2019), the field vole (Microtus agrestis) (Meredith et al. 2015), and the steppe lemming (Lagurus lagurus) (Hofmannová et al. 2014). Domestic small rodents, such as the guinea pigs (Cavia porcellus) are also susceptible to infection (Illanes et al. 1993).
Multifocal granulomatous lesions were identified in association with clinical signs, such as weight loss (Hofmannová et al. 2014; Kitz et al. 2018), behavior changes (Hofmannová et al. 2014), conjunctivitis (Hofmannová et al. 2014), and hind-limb paresis (Hofmannová et al. 2014), and the infection was even fatal for some small rodents (Hofmannová et al. 2014; Kitz et al. 2018).
In experimental mice, albendazole showed reduction of microsporidia burden (Kotkova et al. 2013; Lallo et al. 2013; Sak et al. 2017) and survival prolongation (Sak et al. 2017), becoming the drug of choice in the treatment of rodents.
Small companion animals: cats and dogs
There is still limited information about the course of the disease in cats and dogs, which is believed to be underdiagnosed in these species. Snowden et al. (2009), for example, described several reasons that can be responsible for the lack of diagnosis in dogs: (1) inadequate exploration of the cause of death in young dogs through necropsy, (2) incorrect attribution of neurological signs to more frequent viral diseases in puppies (e.g., canine distemper), and (3) unavailability of routine canine serological screening tests for E. cuniculi. In addition, we would add another potential reason: lack of knowledge and awareness of small animal veterinarians for this disease, a paradigm that we intend to change with this review.
Nevertheless, it is currently known that both species can be infected with E. cuniculi through oral ingestion of water or food contaminated with spores and that they can develop clinically relevant and potentially fatal disease (Snowden et al. 2009; Postma et al. 2018; Addie et al. 2020). Moreover, stray and wild animals are at greater risk of infection, compared to domestic ones, since the exposure in the outdoor environment is significantly superior (Lindsay et al. 2009; Addie et al. 2020).
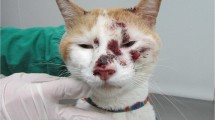
The main clinical manifestation of a symptomatic infected cat is the development of ocular abnormalities, such as cataracts and anterior uveitis (Benz et al. 2011; Künzel et al. 2014; Addie et al. 2020). Thus, the therapeutic protocol for cats with ocular lesions due to parasitic infection often involves the surgical removal of the affected lens (phacoemulsification), in addition to the administration of fenbendazole (20 mg/kg daily once daily for 21 consecutive days) and the symptomatic treatment of uveitis (Benz et al. 2011; Addie et al. 2020).
On the other hand, canine patients with symptomatic infection normally develop systemic abnormalities compatible with the so-called encephalitis-nephritis syndrome, presenting neurological changes, such as epileptic seizures (Postma et al. 2018; Boer et al. 2021), altered mental state (Snowden et al. 2009; Postma et al. 2018), ataxia (Snowden et al. 2009; Boer et al. 2021), tremors (Boer et al. 2021), circling (Snowden et al. 2009), and central blindness (Snowden et al. 2009), but also anorexia and weight loss (Snowden et al. 2009; Postma et al. 2018). However, Nell et al. (2015) reported ocular lesions similar to those found in cats infected with E. cuniculi. In that case report, three dogs aged between 6 months and 7 and a half years were diagnosed with chronic anterior uveitis and focal anterior cortical cataract (Nell et al. 2015). As mentioned for cats, the same benzimidazole drug (fenbendazole) has been prescribed in dogs at doses ranging from 25 to 55 mg/kg once daily for 10 to 30 consecutive days, depending on their therapeutic response, along with the supportive and symptomatic treatment (Nell et al. 2015; Boer et al. 2021). In the three dogs with the described ocular lesions, the phacoemulsification technique was also elected for their treatment with satisfactory results (Nell et al. 2015).
Humans
The emergence of patients diagnosed with acquired immunodeficiency syndrome (AIDS) in the 1980s marked the knowledge about this opportunistic parasitosis in humans, since until this time, E. cuniculi was rarely diagnosed in people. The main reason for this epidemiological association is the impairment of the immune system of these individuals that allowed the clinical manifestation of the infection (Didier 2005; Didier and Weiss 2006). Beyond that, homosexual practice among these patients proved to be an increased risk factor of parasite transmission, with microsporidia detected in the urethra and prostate (Didier 2005). Nevertheless, the increasing prescription of combination antiretroviral therapy to these patients has reduced the occurrence of this opportunistic infection. However, in less developed countries, where this therapy is less available, the infection continues to have a worrying prevalence in these immunocompromised patients (Didier and Weiss 2011).
In addition to AIDS patients, other groups of people are considered to be at risk such as cancer patients undergoing chemotherapy, the elderly and children, diabetic patients, and travelers (Didier and Weiss 2011), but also organ transplant recipients (Talabani et al. 2010; Didier and Weiss 2011; Hocevar et al. 2014; Ladapo et al. 2014; Kicia et al. 2019), employees of slaughterhouse (Halánová et al. 2003), and animal care workers (Ozkan et al. 2011; Sak et al. 2011b; Carhan et al. 2015). Moreover, in a recent review, the potential zoonotic risk for patients undergoing animal-assisted interventions was highlighted, due to the close and continued physical contact that these therapies often require (Santaniello et al. 2021).
Therefore, E. cuniculi infection in humans has been associated mainly with immunocompromised individuals, in whom higher percentages of seropositivity have been reported (Halánová et al. 2003; Abu-Akkada et al. 2015b). The development of clinical disease is particularly related to these patients, with the manifestation of different symptoms, which may even lead to death (De Groote et al. 1995; Weber et al. 1997; Fournier et al. 2000; Kicia et al. 2019). In contrast, the infection is mostly subclinical in immunocompetent humans (Sak et al. 2011a).
E. cuniculi can infect and spread to various organs, causing multiple syndromes, such as encephalitis, keratoconjunctivitis, nephritis, hepatitis, myositis, peritonitis, sinusitis, and pneumonia (Didier and Weiss 2011). The main clinical signs detected in humans with microsporidia are fever of unknown origin (De Groote et al. 1995; Talabani et al. 2010; Ladapo et al. 2014; Kicia et al. 2019), diarrhea (Weber et al. 1997; Didier and Weiss 2011; Ladapo et al. 2014), and ocular (De Groote et al. 1995; Franzen et al. 1995; Fournier et al. 2000), nasal (De Groote et al. 1995; Franzen et al. 1995), and respiratory signs (De Groote et al. 1995; Talabani et al. 2010; Kicia et al. 2019), in addition to more nonspecific signs, such as anorexia, wasting, and weight loss (De Groote et al. 1995; Fournier et al. 2000; Talabani et al. 2010; Didier and Weiss 2011). When E. cuniculi reaches the brain tissue, other clinical manifestations, such as headache and visual and cognitive impairments, may be present (Weber et al. 1997).
Albendazole is the drug of choice for the treatment of E. cuniculi in humans and has been prescribed at the dose of 200–400 mg twice daily (De Groote et al. 1995; Franzen et al. 1995; Weber et al. 1997; Talabani et al. 2010; Hocevar et al. 2014; Ladapo et al. 2014; Carhan et al. 2015).The duration of treatment depends on the resolution of clinical signs, but more objectively on the spore clearance in the urine (Weber et al. 1997; Hocevar et al. 2014; Ladapo et al. 2014; Carhan et al. 2015) and also on the time required for the white cell counts (mostly CD4 + cells) to rise to values considered acceptable for the health of the individual (Talabani et al. 2010).
Infection in other animal species: particularities and potential impact on the ecosystem
Horses
Levkutová et al. (2004) described a seroprevalence of 59.7% and 83.3%, respectively, in 72 asymptomatic and 30 symptomatic horses from Israel, while Goodwin et al. (2006) reported a seroprevalence between 12.5–14.1% in 559 horses from Brazil. In contrast, Cray et al. (2014) showed that only 4.8% of 105 clinically abnormal horses from USA were seropositive. Although this percentage result is comparatively lower in comparison with the previous studies, it is in line with the value reported by Wagnerová et al. (2012) in horses from the Czech Republic, through the PCR detection of E. cuniculi in their fecal samples: 6.9% of 377 horses.
In this species, E. cuniculi infection in pregnant mares has been associated with placentitis (Patterson-Kane et al. 2003) and abortion (Patterson-Kane et al. 2003; Szeredi et al. 2007). Moreover, other clinical signs, such as colic (Levkutová et al. 2004; Cray et al. 2014), fever of unknown origin (Levkutová et al. 2004; Cray et al. 2014), and neurological signs (Levkutová et al. 2004), were reported in seropositive horses, although it was not possible to confirm a clear correlation between these clinical manifestations and seropositivity (Levkutová et al. 2004; Cray et al. 2014).
Production animals
The presence of E. cuniculi among production and farm animals has been reported, usually associated with subclinical infection (Abu-Akkada et al. 2015a). Previous serological studies showed a percentage of seropositivity of 67.7% in goats (Abu-Akkada et al. 2015a), 52.2% in swine (Malčeková et al. 2010), 46.4% in buffaloes (Abu-Akkada et al. 2015a), 2.4–28.1% in cattle (Malčeková et al. 2010; Abu-Akkada et al. 2015a), and 8.6–13.6% in sheep (Halánová et al. 2003; Malčeková et al. 2010; Abu-Akkada et al. 2015a). E. cuniculi was also identified in urine and fecal samples of cattle (Abu-Akkada et al. 2015a; Kváč et al. 2016) and swine (Reetz et al. 2009; Sak et al. 2019), through molecular surveys. According to Kvapil et al. (2021), herbivores seem to be more likely to become infected with E. cuniculi than carnivores and omnivores.
Wild, exotic, and zoological mammal species
Wild species have been considered as potential reservoirs of infections to domestic animals and even humans, based on serological studies. Therefore, antibodies against E. cuniculi have been detected in several wild carnivores, such as the red fox (Vulpes vulpes) (Meredith et al. 2015), the arctic/blue fox (Alopex lagopus) (Hersteinsson et al. 1993; Akerstedt 2002), and the feral mink (Mustela vison) (Hersteinsson et al. 1993), but also in wild lagomorphs like European hares (Lepus europaeus) (Bártová et al. 2015; Özkan et al. 2021). Given the epidemiological results of recent studies, seroprevalence appears to be higher in foxes (52.1%) (Meredith et al. 2015) than in hares (0.47–2.9%) (Bártová et al. 2015; Özkan et al. 2021). Most of these wild animals are asymptomatic carriers; however, cases of infected arctic/blue fox pups with neurological signs have been described (Hersteinsson et al. 1993; Mathis et al. 1996).
In relation to exotic or zoological species, studies carried out in captive mammal animals from zoos and circuses described a seroprevalence of antibodies against E. cuniculi ranging from 20 to 44% (Marková et al. 2019; Kvapil et al. 2021). In these epidemiological surveys, a wide variety of seropositive animals were found, including the alpaca (Lama guanicoe f. pacos) (Marková et al. 2019; Kvapil et al. 2021), the bactrian camel (Camelus bactrianus) (Marková et al. 2019; Kvapil et al. 2021), the dromedary camel (Camelus dromedarius) (Marková et al. 2019), the llama (Lama glama) (Marková et al. 2019), the brown bear (Ursus arctos) (Kvapil et al. 2021), and the Euroasian lynx (Lynx lynx) (Kvapil et al. 2021).
The presence of E. cuniculi among this species has also been confirmed by PCR-based techniques, which allowed, for example, the detection in fecal samples of clinically healthy great apes from European zoos and African sanctuaries, such as chimpanzees (Pan troglodytes), bonobos (Pan paniscus), and gorillas (Gorilla gorilla) (Sak et al. 2011b); in the brain tissue of two stone martens (Martes foina) and a European otter (Lutra lutra) (Hůrková and Modrý 2006); in the ocular tissue of a snow leopard (Panthera uncia) (Scurrell et al. 2015); in the tissue specimens of the major organ systems of captive emperor tamarins (Saguinus imperator) (Guscetti et al. 2003); and in the brain, lung, and bone marrow specimens from cotton-top tamarins (Saguinus oedipus) (Juan-Sallés et al. 2006).
Although most exotic and zoological animals usually present subclinical infection by E. cuniculi, some individuals showed clinical signs [e.g., phacoclastic uveitis in a snow leopard (Scurrell et al. 2015)] or in some cases overt disease and death [e.g., as reported in captive emperor and cotton-top tamarins (Guscetti et al. 2003; Juan-Sallés et al. 2006)].
Birds
Birds species are also susceptible of E. cuniculi infection, despite being mostly asymptomatic, as described in previous epidemiological surveys (Kašičková et al. 2009; Abu-Akkada 2019).
A study carried out in the Czech Republic confirmed the presence of E. cuniculi by PCR detection in the feces of 287 exotic birds, showing an overall prevalence of 12.5% (Kašičková et al. 2009). This epidemiological survey allowed the detection of this pathogen in individuals of the orders Psittaciformes and Passeriformes, such as the rosy-faced lovebird (Agapornis roseicollis), the budgerigar (Melopsittacus undulatus), and the canary (Serinus canaria). Moreover, a serological screening in raised chicken detected antibodies against E. cuniculi in 14.8% of 88 animals examined (Abu-Akkada 2019).
Reptiles
Microsporidia are rarely identified in reptiles, but some authors have already described its presence in the Inland Bearded Dragon (Pogona vitticeps), an increasingly pet animal. Contrary to what was initially thought, the pathogen that affects this species is not E. cuniculi, but rather a new species named Encephalitozoon pogonae (Sokolova et al. 2016). In fact, although there is a significant similarity between the morphology and pathogenesis of the two agents, their differentiation was possible through the evaluation of the ITS region as, at this level, the E. pogonae has two repeats of the sequence 5′-GTTT-3′ in two different places (Richter et al. 2013). Clinically, affected animals show granulomatous lesions in different regions, such as joints, heart, adrenal glands, liver, spleen, kidneys, and gastrointestinal mucosa (Richter et al. 2013; Sokolova et al. 2016). Further studies are needed for the detection of this parasite in other reptile species, especially wild ones, which lack investigation as noted in the current literature.
Prophylaxis and infection control measures
As rabbits are the natural host for E. cuniculi, most prophylactic and control measures are directed to this species. One of the main recommended measures is serological screening, in order to separate seropositive from seronegative rabbits and to prevent the transmission of E. cuniculi among colonies (Fukui et al. 2013). In fact, if circulating antibodies against E. cuniculi are early detected in a given animal, it is even possible to prevent that animal from contaminating the environment and other animals, since they can be detected 3–4 weeks before the appearance of spores in the urine (Deplazes et al. 2000). Therefore, it has been suggested that all rabbits should be screened prior to introduction into new colonies to prevent the entrance of E. cuniculi, which can potentially lead to an outbreak of new infections (Okewole 2008; Fukui et al. 2013). Moreover, serological screening examinations have been recommended for pet rabbits whose owners have a less competent immune system, such as AIDS patients, children, and the elderly, as they are at greater risk of becoming infected and developing clinical disease (Dipineto et al. 2008).
According to this point of view, if an outbreak is detected, the effective approach of “all-out and all-in” may be implemented at the animal production systems (Fukui et al. 2013).
Another measure described to prevent the development of the disease is the administration of fenbendazole not only for the treatment of infected rabbits, but also for rabbits at risk, since this drug proved to prevent the persistence of E. cuniculi in the animal’s body when administered before its exposure (Suter et al. 2001). Associated with the previous measures, other important precaution is to keep rabbits in a stable and stress-free condition, since changes in the environment seem to be a triggering element for the development of clinical signs in previously infected rabbits (Künzel and Fisher 2018; Dalboni et al. 2021).
In terms of prophylaxis, although one study described that subcutaneous immunization with inactivated spores of E. cuniculi stimulates humoral immunity enough to produce a “highly active rabbit hyperimmune sera” against this pathogen (Sobottka et al. 2001), there are still no vaccine products available for this purpose. More studies are needed to confirm the feasibility and effectiveness of this type of immunization.
In humans, and considering the zoonotic risk for people who work with animals, staff education should be encouraged (Fukui et al. 2013) and personal hygiene should be ensured for those who handle the animals and who eventually come into contact with potential sources of infection, such as their urine, for example (Ozkan et al. 2011; Carhan et al. 2015).
Another effective measure to reduce the potential exposure of animals and humans to E. cuniculi spores is the regular cleaning and disinfection of animal housing facilities with bleach and 70% ethanol, which showed high efficacy in killing spores even after a short contact time (Jordan et al. 2006a).
Conclusion
In light of the present review, we concluded that regular serological screening is essential for the early detection of infected individuals and to identify the prevalence and incidence in numerous species and geographic locations; animal and human health professionals should be more aware and educated to diagnose this disease, considering it in cases of neurological, ocular, and/or renal conditions refractory to treatment or when other more frequent diseases have already been discarded; immunocompromised people must reinforce care and personal protective measures, avoiding the aforementioned risk factors, especially if they come into contact with lagomorphs; and the general public should be more conscious about the existence of this parasite in the environment, promoting personal and community hygiene practices and ensuring the protection of public health.
We also detected some gaps in the literature that could be considered as research line opportunities in the future such as the evaluation of this pathogen in wild species, as the sylvatic life cycle is not yet fully understood and may be crucial to eradicate this parasite in some geographic areas. Moreover, we also suggest the serological assessment of other risk groups susceptible to infection, in addition to immunocompromised people, such as breeders and veterinarians, who may be potential asymptomatic carriers due to the high and continued exposure to which they are subjected. Finally, in our view, it is also necessary to continue to evaluate the applicability, sensitivity, and specificity of the current screening tests and even to consider new, faster, and more practical approaches to facilitate the evaluation of different free-ranging animals that can act as reservoirs.
Therefore, we showed that E. cuniculi is currently present worldwide and circulates in several animal and human populations, which makes it a public health problem that needs to be addressed in the one health context due to its low host specificity, its high resistance in the environment, and the possibility of causing serious disease or even death, depending on the individual’s health status.
Data availability
All data and material used in this review will be made available whenever requested.
References
Abu-Akkada SS (2019) First finding of antibodies to Encephalitozoon cuniculi in raised chickens in Egypt. Egypt Vet Med Soc Parasitol J (EVMSPJ) 15(1):20–28. https://doi.org/10.21608/evmspj.2019.80817
Abu-Akkada SS, Ashmawy KI, Dweir AW (2015) First detection of an ignored parasite, Encephalitozoon cuniculi, in different animal hosts in Egypt. Parasitol Res 114(3):843–850. https://doi.org/10.1007/s00436-014-4247-4
Abu-Akkada SS et al (2015) Encephalitozoon cuniculi infection among immunocompromised and immunocompetent humans in Egypt. Iran J Parasitol 10(4):561–570
Addie DD et al (2020) Encephalitozoon cuniculi infection in cats: European guidelines from the ABCD on prevention and management. J Feline Med Surg 22(11):1084–1088. https://doi.org/10.1177/1098612x20941787
Akerstedt J (2002) An indirect ELISA for detection of Encephalitozoon cuniculi infection in farmed blue foxes (Alopex lagopus). Acta Vet Scand 43(4):211–220. https://doi.org/10.1186/1751-0147-43-211
Akerstedt J (2003) Serological investigation of canine encephalitozoonosis in Norway. Parasitol Res 89(1):49–52. https://doi.org/10.1007/s00436-002-0724-2
Ashmawy KI, Abuakkada SS, Awad AM (2011) Seroprevalence of antibodies to Encephalitozoon cuniculi and Toxoplasma gondii in farmed domestic rabbits in Egypt. Zoonoses Public Health 58(5):357–364. https://doi.org/10.1111/j.1863-2378.2010.01371.x
Baneux PJ, Pognan F (2003) In utero transmission of Encephalitozoon cuniculi strain type I in rabbits. Lab Anim 37(2):132–138. https://doi.org/10.1258/00236770360563778
Bártová E, Marková J, Sedlák K (2015) Prevalence of antibodies to Encephalitozoon cuniculi in European hares (Lepus europaeus). Ann Agric Environ Med 22(4):674–676. https://doi.org/10.5604/12321966.1185773
Benz P et al (2011) Detection of Encephalitozoon cuniculi in the feline cataractous lens. Vet Ophthalmol 14(Suppl 1):37–47. https://doi.org/10.1111/j.1463-5224.2011.00873.x
Berger Baldotto S, Cray C, Giannico AT, Reifur L, Montiani-Ferreira F (2015) Seroprevalence of Encephalitozoon cuniculi infection in pet rabbits in Brazil. J Exotic Pet Med 24(4):435–440. https://doi.org/10.1053/j.jepm.2015.08.010
Boer TSd, Diaz Espineira MM, Mandigers PJJ (2021) Is Encephalitozoon cuniculi of significance in young dogs with neurological signs? Front Vet Sci 8:678968. https://doi.org/10.3389/fvets.2021.678968
Bohne W, Böttcher K, Gross U (2011) The parasitophorous vacuole of Encephalitozoon cuniculi: biogenesis and characteristics of the host cell-pathogen interface. Int J Med Microbiol 301(5):395–399. https://doi.org/10.1016/j.ijmm.2011.04.006
Boot R, Hansen AK, Hansen CK, Nozari N, Thuis HC (2000) Comparison of assays for antibodies to Encephalitozoon cuniculi in rabbits. Lab Anim 34(3):281–289. https://doi.org/10.1258/002367700780384726
Carhan A, Ozkan O, Ozkaya E (2015) The first identification of Encephalitozoon cuniculi infection in an animal care worker in Turkey. Iran J Parasitol 10(2):280–285
Cox JC, Hamilton RC, Attwood HD (1979) An investigation of the route and progression of Encephalitozoon cuniculi infection in adult rabbits. J Protozool 26(2):260–265. https://doi.org/10.1111/j.1550-7408.1979.tb02776.x
Cray C, Rivas Y (2013) Seroprevalence of Encephalitozoon cuniculi in dogs in the United States. J Parasitol 99(1):153–154. https://doi.org/10.1645/ge-3152.1
Cray C, Perritt E, Hughes C, Belgrave RL (2014) Serological survey for antibody to Encephalitozoon cuniculi in horses in the USA. Parasitol Res 113(7):2757–2759. https://doi.org/10.1007/s00436-014-3930-9
Csokai J, Joachim A, Gruber A, Tichy A, Pakozdy A, Künzel F (2009) Diagnostic markers for encephalitozoonosis in pet rabbits. Vet Parasitol 163(1–2):18–26. https://doi.org/10.1016/j.vetpar.2009.03.057
Dalboni LC et al (2021) Encephalitozoon cuniculi takes advantage of efferocytosis to evade the immune response. PLoS One 16(3):e0247658. https://doi.org/10.1371/journal.pone.0247658
De Groote MA et al (1995) Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis 171(5):1375–1378. https://doi.org/10.1093/infdis/171.5.1375
Deplazes P, Mathis A, Baumgartner R, Tanner I, Weber R (1996) Immunologic and molecular characteristics of Encephalitozoon-like microsporidia isolated from humans and rabbits indicate that Encephalitozoon cuniculi is a zoonotic parasite. Clin Infect Dis 22(3):557–559. https://doi.org/10.1093/clinids/22.3.557
Deplazes P, Mathis A, Weber R (2000) Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib Microbiol 6:236–260. https://doi.org/10.1159/000060363
Desoubeaux G, Pantin A, Peschke R, Joachim A, Cray C (2017) Application of Western blot analysis for the diagnosis of Encephalitozoon cuniculi infection in rabbits: example of a quantitative approach. Parasitol Res 116(2):743–750. https://doi.org/10.1007/s00436-016-5343-4
Didier ES (2005) Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop 94(1):61–76. https://doi.org/10.1016/j.actatropica.2005.01.010
Didier ES, Weiss LM (2006) Microsporidiosis: current status. Curr Opin Infect Dis 19(5):485–492. https://doi.org/10.1097/01.qco.0000244055.46382.23
Didier ES, Weiss LM (2011) Microsporidiosis: not just in AIDS patients. Curr Opin Infect Dis 24(5):490–495. https://doi.org/10.1097/QCO.0b013e32834aa152
Didier ES, Vossbrinck CR, Baker MD, Rogers LB, Bertucci DC, Shadduck JA (1995) Identification and characterization of three Encephalitozoon cuniculi strains. Parasitology 111(Pt 4):411–421. https://doi.org/10.1017/s0031182000065914
Dipineto L et al (2008) Serological survey for antibodies to Encephalitozoon cuniculi in pet rabbits in Italy. Zoonoses Public Health 55(3):173–175. https://doi.org/10.1111/j.1863-2378.2007.01097.x
Fasshauer V, Gross U, Bohne W (2005) The parasitophorous vacuole membrane of Encephalitozoon cuniculi lacks host cell membrane proteins immediately after invasion. Eukaryot Cell 4(1):221–224. https://doi.org/10.1128/ec.4.1.221-224.2005
Fournier S et al (2000) Disseminated infection due to Encephalitozoon cuniculi in a patient with AIDS: case report and review. HIV Med 1(3):155–161. https://doi.org/10.1046/j.1468-1293.2000.00022.x
Franzen C et al (1995) Immunologically confirmed disseminated, asymptomatic Encephalitozoon cuniculi infection of the gastrointestinal tract in a patient with AIDS. Clin Infect Dis 21(6):1480–1484. https://doi.org/10.1093/clinids/21.6.1480
Fukui D et al (2013) Surveillance for an outbreak of Encephalitozoon cuniculi infection in rabbits housed at a zoo and biosecurity countermeasures. J Vet Med Sci 75(1):55–61. https://doi.org/10.1292/jvms.12-0231
Furuya K, Asakura T, Igarashi M, Morita T (2009) Microsporidian Encephalitozoon cuniculi antibodies in rabbit urine samples. Vet Rec 165(3):85–86. https://doi.org/10.1136/vetrec.165.3.85
Giordano C, Weigt A, Vercelli A, Rondena M, Grilli G, Giudice C (2005) Immunohistochemical identification of Encephalitozoon cuniculi in phacoclastic uveitis in four rabbits. Vet Ophthalmol 8(4):271–275. https://doi.org/10.1111/j.1463-5224.2005.00394.x
Goodwin D, Gennari SM, Howe DK, Dubey JP, Zajac AM, Lindsay DS (2006) Prevalence of antibodies to Encephalitozoon cuniculi in horses from Brazil. Vet Parasitol 142(3–4):380–382. https://doi.org/10.1016/j.vetpar.2006.07.006
Graham JE, Garner MM, Reavill DR (2014) Benzimidazole toxicosis in rabbits: 13 Cases (2003 to 2011). J Exotic Pet Med 23(2):188–195. https://doi.org/10.1053/j.jepm.2014.02.012
Guscetti F, Mathis A, Hatt JM, Deplazes P (2003) Overt fatal and chronic subclinical Encephalitozoon cuniculi microsporidiosis in a colony of captive emperor tamarins (Saguinus imperator). J Med Primatol 32(2):111–119. https://doi.org/10.1034/j.1600-0684.2003.00016.x
Halánová M, Cisláková L, Valencákova A, Bálent P, Adam J, Trávnicek M (2003) Serological screening of occurrence of antibodies to Encephalitozoon cuniculi in humans and animals in Eastern Slovakia. Ann Agric Environ Med 10(1):117–120
Halánová M et al (2013) Occurrence of microsporidia as emerging pathogens in Slovak Roma children and their impact on public health. Ann Agric Environ Med 20(4):695–698
Hein J, Flock U, Sauter-Louis C, Hartmann K (2014) Encephalitozoon cuniculi in rabbits in Germany: prevalence and sensitivity of antibody testing. Vet Rec 174(14):350. https://doi.org/10.1136/vr.102126
Hersteinsson P, Gunnarsson E, Hjartardóttir S, Skírnisson K (1993) Prevalence of Encephalitozoon cuniculi antibodies in terrestrial mammals in Iceland, 1986 to 1989. J Wildl Dis 29(2):341–344. https://doi.org/10.7589/0090-3558-29.2.341
Hocevar SN et al (2014) Microsporidiosis acquired through solid organ transplantation: a public health investigation. Ann Intern Med 160(4):213–220. https://doi.org/10.7326/m13-2226
Hofmannová L, Sak B, Jekl V, Mináriková A, Skorič M, Kváč M (2014) Lethal Encephalitozoon cuniculi genotype III infection in Steppe lemmings (Lagurus lagurus). Vet Parasitol 205(1–2):357–360. https://doi.org/10.1016/j.vetpar.2014.07.008
Hsu V, Grant DC, Zajac AM, Witonsky SG, Lindsay DS (2011) Prevalence of IgG antibodies to Encephalitozoon cuniculi and Toxoplasma gondii in cats with and without chronic kidney disease from Virginia. Vet Parasitol 176(1):23–26. https://doi.org/10.1016/j.vetpar.2010.10.022
Hůrková L, Modrý D (2006) PCR detection of Neospora caninum, Toxoplasma gondii and Encephalitozoon cuniculi in brains of wild carnivores. Vet Parasitol 137(1–2):150–154. https://doi.org/10.1016/j.vetpar.2006.01.005
Igarashi M, Oohashi E, Dautu G, Ueno A, Kariya T, Furuya K (2008) High seroprevalence of Encephalitozoon cuniculi in pet rabbits in Japan. J Vet Med Sci 70(12):1301–1304. https://doi.org/10.1292/jvms.70.1301
Illanes OG, Tiffani-Castiglioni E, Edwards JF, Shadduck JA (1993) Spontaneous encephalitozoonosis in an experimental group of guinea pigs. J Vet Diagn Invest 5(4):649–651. https://doi.org/10.1177/104063879300500432
Jass A, Matiasek K, Henke J, Küchenhoff H, Hartmann K, Fischer A (2008) Analysis of cerebrospinal fluid in healthy rabbits and rabbits with clinically suspected encephalitozoonosis. Vet Rec 162(19):618–622. https://doi.org/10.1136/vr.162.19.618
Jeklova E et al (2010) Usefulness of detection of specific IgM and IgG antibodies for diagnosis of clinical encephalitozoonosis in pet rabbits. Vet Parasitol 170(1–2):143–148. https://doi.org/10.1016/j.vetpar.2010.01.029
Jeklova E, Leva L, Matiasovic J, Ondrackova P, Kummer V, Faldyna M (2020) Characterization of humoral and cell-mediated immunity in rabbits orally infected with Encephalitozoon cuniculi. Vet Res 51(1):79. https://doi.org/10.1186/s13567-020-00806-9
Jordan CN, Dicristina JA, Lindsay DS (2006) Activity of bleach, ethanol and two commercial disinfectants against spores of Encephalitozoon cuniculi. Vet Parasitol 136(3–4):343–346. https://doi.org/10.1016/j.vetpar.2005.11.005
Jordan CN, Zajac AM, Snowden KS, Lindsay DS (2006) Direct agglutination test for Encephalitozoon cuniculi. Vet Parasitol 135(3–4):235–240. https://doi.org/10.1016/j.vetpar.2005.09.009
Juan-Sallés C et al (2006) Disseminated encephalitozoonosis in captive, juvenile, cotton-top (Saguinus oedipus) and neonatal emperor (Saguinus imperator) tamarins in North America. Vet Pathol 43(4):438–446. https://doi.org/10.1354/vp.43-4-438
Kašičková D, Sak B, Kváč M, Ditrich O (2009) Sources of potentially infectious human microsporidia: molecular characterisation of microsporidia isolates from exotic birds in the Czech Republic, prevalence study and importance of birds in epidemiology of the human microsporidial infections. Vet Parasitol 165(1):125–130. https://doi.org/10.1016/j.vetpar.2009.06.033
Keeble EJ, Shaw DJ (2006) Seroprevalence of antibodies to Encephalitozoon cuniculi in domestic rabbits in the United Kingdom. Vet Rec 158(16):539–544. https://doi.org/10.1136/vr.158.16.539
Khan IA, Moretto M, Weiss LM (2001) Immune response to Encephalitozoon cuniculi infection. Microbes Infect 3(5):401–405. https://doi.org/10.1016/s1286-4579(01)01397-1
Kicia M et al (2019) Symptomatic respiratory Encephalitozoon cuniculi infection in renal transplant recipients. Int J Infect Dis 79:21–25. https://doi.org/10.1016/j.ijid.2018.10.016
Kitz S, Grimm F, Wenger S, Hatt J, Kipar A, Hetzel U (2018) Encephalitozoon cuniculi infection in Barbary striped grass mice (Lemniscomys barbarus). Schweiz Arch Tierheilkd 160(6):394–400. https://doi.org/10.17236/sat00166
Kotkova M, Sak B, Kvetonova D, Kvac M (2013) Latent microsporidiosis caused by Encephalitozoon cuniculi in immunocompetent hosts: a murine model demonstrating the ineffectiveness of the immune system and treatment with albendazole. PLoS One 8(4):e60941. https://doi.org/10.1371/journal.pone.0060941
Kotková M, Sak B, Hlásková L, Květoňová D, Kváč M (2018) Evidence of transplacental transmission of Encephalitozoon cuniculi genotype II in murine model. Exp Parasitol 193:51–57. https://doi.org/10.1016/j.exppara.2018.09.001
Kourgelis C, Reilly C, Von Roedern M, Cray C (2017) Serological survey for antibodies to Encephalitozoon cuniculi in cats within the United States. Vet Parasitol Reg Stud Reports 9:122–124. https://doi.org/10.1016/j.vprsr.2017.06.009
Künzel F, Fisher PG (2018) Clinical signs, diagnosis, and treatment of Encephalitozoon cuniculi infection in rabbits. Vet Clin North Am Exot Anim Pract 21(1):69–82. https://doi.org/10.1016/j.cvex.2017.08.002
Künzel F et al (2008) Clinical symptoms and diagnosis of encephalitozoonosis in pet rabbits. Vet Parasitol 151(2–4):115–124. https://doi.org/10.1016/j.vetpar.2007.11.005
Künzel F, Peschke R, Tichy A, Joachim A (2014) Comparison of an indirect fluorescent antibody test with Western blot for the detection of serum antibodies against Encephalitozoon cuniculi in cats. Parasitol Res 113(12):4457–4462. https://doi.org/10.1007/s00436-014-4130-3
Kváč M et al (2016) Encephalitozoon cuniculi in raw cow’s milk remains infectious after pasteurization. Foodborne Pathog Dis 13(2):77–79. https://doi.org/10.1089/fpd.2015.2036
Kvapil P et al (2021) Biosurveillance of selected pathogens with zoonotic potential in a zoo. Pathogens 10(4):428. https://doi.org/10.3390/pathogens10040428
Ladapo TA et al (2014) Microsporidiosis in pediatric renal transplant patients in Cape Town, South Africa: two case reports. Pediatr Transplant 18(7):E220–E226. https://doi.org/10.1111/petr.12327
Lallo MA, da Costa LFV, de Castro JM (2013) Effect of three drugs against Encephalitozoon cuniculi infection in immunosuppressed mice. Antimicrob Agents Chemother 57(7):3067–3071. https://doi.org/10.1128/AAC.00157-13
Latney LV, Bradley CW, Wyre NR (2014) Encephalitozoon cuniculi in pet rabbits: diagnosis and optimal management. Vet Med (auckl) 5:169–180. https://doi.org/10.2147/vmrr.S49842
Lavazza A, Chiari M, Nassuato C, Giardiello D, Tittarelli C, Grilli G (2016) Serological investigation on Encephalitozoon cuniculi in pet rabbits in North-Central Italy. J Exotic Pet Med 25(1):52–59. https://doi.org/10.1053/j.jepm.2015.12.004
Leipig M et al (2013) Value of histopathology, immunohistochemistry, and real-time polymerase chain reaction in the confirmatory diagnosis of Encephalitozoon cuniculi infection in rabbits. J Vet Diagn Invest 25(1):16–26. https://doi.org/10.1177/1040638712466394
Levkutová M, Hípiková V, Faitelzon S, Benath G, Paulík S, Levkut M (2004) Prevalence of antibodies to Encephalitozoon cuniculi in horses in the Israel. Ann Agric Environ Med 11(2):265–267
Li X, Palmer R, Trout JM, Fayer R (2003) Infectivity of microsporidia spores stored in water at environmental temperatures. J Parasitol 89(1):185–188. https://doi.org/10.1645/0022-3395(2003)089[0185:Iomssi]2.0.Co;2
Lindsay DS et al (2009) Serological survey for antibodies to Encephalitozoon cuniculi in ownerless dogs from urban areas of Brazil and Colombia. J Parasitol 95(3):760–763. https://doi.org/10.1645/ge-1890.1
Lobo ML et al (2003) Microsporidia detection in stools from pets and animals from the zoo in Portugal: a preliminary study. J Eukaryot Microbiol 50(Suppl):581–582. https://doi.org/10.1111/j.1550-7408.2003.tb00638.x
Lonardi C, Grilli G, Ferrazzi V, Dal Cin M, Rigolin D, Piccirillo A (2013) Serological survey of Encephalitozoon cuniculi infection in commercially reared rabbit does in Northern Italy. Res Vet Sci 94(2):295–298. https://doi.org/10.1016/j.rvsc.2012.09.020
Maestrini G et al (2017) Encephalitozoon cuniculi in rabbits: serological screening and histopathological findings. Comp Immunol Microbiol Infect Dis 50:54–57. https://doi.org/10.1016/j.cimid.2016.11.012
Malčeková B et al (2010) Seroprevalence of antibodies to Encephalitozoon cuniculi and Encephalitozoon intestinalis in humans and animals. Res Vet Sci 89(3):358–361. https://doi.org/10.1016/j.rvsc.2010.03.020
Marková J et al (2019) Toxoplasma gondii, Neospora caninum and Encephalitozoon cuniculi in animals from captivity (zoo and circus animals). J Eukaryot Microbiol 66(3):442–446. https://doi.org/10.1111/jeu.12688
Mathis A, Akerstedt J, Tharaldsen J, Odegaard O, Deplazes P (1996) Isolates of Encephalitozoon cuniculi from farmed blue foxes (Alopex lagopus) from Norway differ from isolates from Swiss domestic rabbits (Oryctolagus cuniculus). Parasitol Res 82(8):727–730. https://doi.org/10.1007/s004360050192
Mathis A, Weber R, Deplazes P (2005) Zoonotic potential of the microsporidia. Clin Microbiol Rev 18(3):423–445. https://doi.org/10.1128/CMR.18.3.423-445.2005
Meredith AL, Cleaveland SC, Brown J, Mahajan A, Shaw DJ (2015) Seroprevalence of Encephalitozoon cuniculi in wild rodents, foxes and domestic cats in three sites in the United Kingdom. Transbound Emerg Dis 62(2):148–156. https://doi.org/10.1111/tbed.12091
Mo L, Drancourt M (2004) Monoclonal antibodies for specific detection of Encephalitozoon cuniculi. Clin Diagn Lab Immunol 11(6):1060–1063. https://doi.org/10.1128/cdli.11.6.1060-1063.2004
Morsy EA, Salem HM, Khattab MS, Hamza DA, Abuowarda MM (2020) Encephalitozoon cuniculi infection in farmed rabbits in Egypt. Acta Vet Scand 62(1):11. https://doi.org/10.1186/s13028-020-0509-6
Murphy TM et al (2007) Study on the prevalence of Toxoplasma gondii and Neospora caninum and molecular evidence of Encephalitozoon cuniculi and Encephalitozoon (Septata) intestinalis infections in red foxes (Vulpes vulpes) in rural Ireland. Vet Parasitol 146(3–4):227–234. https://doi.org/10.1016/j.vetpar.2007.02.017
Nell B, Csokai J, Fuchs-Baumgartinger A, Maaß G (2015) Encephalitozoon cuniculi causes focal anterior cataract and uveitis in dogs. Tierarztl Prax Ausg K Kleintiere Heimtiere 43(5):337–44. https://doi.org/10.15654/tpk-141053
Neumayerová H et al (2014) Seroprevalence of Toxoplasma gondii and Encephalitozoon cuniculi in rabbits from different farming systems. Vet Parasitol 204(3–4):184–190. https://doi.org/10.1016/j.vetpar.2014.04.020
Okewole EA (2008) Seroprevalence of antibodies to Encephalitozoon cuniculi in domestic rabbits in Nigeria. Onderstepoort J Vet Res 75(1):33–38. https://doi.org/10.4102/ojvr.v75i1.85
Ozkan O, Ozkan AT, Zafer K (2011) Encephalitozoonosis in New Zealand rabbits and potential transmission risk. Vet Parasitol 179(1–3):234–237. https://doi.org/10.1016/j.vetpar.2011.02.007
Özkan Ö, Yücesan B, Babür C, Orkun Ö (2021) Seroprevalence of Encephalitozoon cuniculi, Francisella tularensis and Toxoplasma gondii in zoonotic diseases in European hares (Lepus europaesus). Turkiye Parazitol Derg 45(3):171–175. https://doi.org/10.4274/tpd.galenos.2021.7237
Pan Y, Wang S, Liu X, Li R, Sun Y, Gadahi JA (2015) Seroprevalence of Encephalitozoon cuniculi in Humans and Rabbits in China. Iran J Parasitol 10(2):290–295
Patterson-Kane JC, Caplazi P, Rurangirwa F, Tramontin RR, Wolfsdorf K (2003) Encephalitozoon cuniculi placentitis and abortion in a quarterhorse mare. J Vet Diagn Invest 15(1):57–59. https://doi.org/10.1177/104063870301500113
Perec-Matysiak A et al (2019) The opportunistic pathogen Encephalitozoon cuniculi in wild living Murinae and Arvicolinae in Central Europe. Eur J Protistol 69:14–19. https://doi.org/10.1016/j.ejop.2019.02.004
Postma GC et al (2018) Fatal canine encephalitozoonosis in Latin America, first report. Vet Parasitol Reg Stud Reports 11:15–18. https://doi.org/10.1016/j.vprsr.2017.11.007
Rebel-Bauder B, Leschnik M, Maderner A, Url A (2011) Generalized encephalitozoonosis in a young kitten with cerebellar hypoplasia. J Comp Pathol 145(2–3):126–131. https://doi.org/10.1016/j.jcpa.2010.12.009
Reetz J, Nöckler K, Reckinger S, Vargas MM, Weiske W, Broglia A (2009) Identification of Encephalitozoon cuniculi genotype III and two novel genotypes of Enterocytozoon bieneusi in swine. Parasitol Int 58(3):285–292. https://doi.org/10.1016/j.parint.2009.03.002
Richter B et al (2013) Encephalitozoonosis in two inland bearded dragons (Pogona vitticeps). J Comp Pathol 148(2):278–282. https://doi.org/10.1016/j.jcpa.2012.05.009
Rodríguez-Tovar LE et al (2017) Histochemical study of Encephalitozoon cuniculi spores in the kidneys of naturally infected New Zealand rabbits. J Vet Diagn Invest 29(3):269–277. https://doi.org/10.1177/1040638716668559
Rönnebäumer K, Gross U, Bohne W (2008) The nascent parasitophorous vacuole membrane of Encephalitozoon cuniculi is formed by host cell lipids and contains pores which allow nutrient uptake. Eukaryot Cell 7(6):1001–1008. https://doi.org/10.1128/ec.00004-08
Sak B et al (2011) Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J Clin Microbiol 49(3):1064–1070. https://doi.org/10.1128/jcm.01147-10
Sak B et al (2011) Diversity of microsporidia (Fungi: Microsporidia) among captive great apes in European zoos and African sanctuaries: evidence for zoonotic transmission? Folia Parasitologica 58(2):81–86. https://doi.org/10.14411/fp.2011.008
Sak B et al (2019) Experimental Encephalitozoon cuniculi infection acquired from fermented meat products. Foodborne Pathog Dis 16(6):394–398. https://doi.org/10.1089/fpd.2018.2569
Sak B, Brdíčková K, Holubová N, Květoňová D, Hlásková L, Kváč M (2020) Encephalitozoon cuniculi genotype III evinces a resistance to albendazole treatment in both immunodeficient and immunocompetent mice. Antimicrob Agents Chemother 64(5):e00058-e120. https://doi.org/10.1128/AAC.00058-20
Sak B, Kotková M, Hlásková L, Kváč M (2017) Limited effect of adaptive immune response to control encephalitozoonosis. Parasite Immunol 39:e12496. https://doi.org/10.1111/pim.12496
Santaniello A, Dipineto L, Rinaldi L, Menna LF, Cringoli G, Fioretti A (2009) Serological survey of Encephalitozoon cuniculi in farm rabbits in Italy. Res Vet Sci 87(1):67–69. https://doi.org/10.1016/j.rvsc.2008.12.008
Santaniello A et al (2021) Zoonotic risk of Encephalitozoon cuniculi in animal-assisted interventions: laboratory strategies for the diagnosis of infections in humans and animals. Int J Environ Res Public Health 18(17):9333. https://doi.org/10.3390/ijerph18179333
Sasaki M, Yamazaki A, Haraguchi A, Tatsumi M, Ishida K, Ikadai H (2011) Serological survey of Encephalitozoon cuniculi infection in Japanese dogs. J Parasitol 97(1):167–169. https://doi.org/10.1645/ge-2540.1
Scurrell EJ et al (2015) Bilateral lenticular Encephalitozoon cuniculi infection in a snow leopard (Panthera uncia). Vet Ophthalmol 18(s1):143–147. https://doi.org/10.1111/vop.12218
Shin JC, Kim DG, Kim SH, Kim S, Song KH (2014) Seroprevalence of Encephalitozoon cuniculi in pet rabbits in Korea. Korean J Parasitol 52(3):321–323. https://doi.org/10.3347/kjp.2014.52.3.321
Snowden KF, Lewis BC, Hoffman J, Mansell J (2009) Encephalitozoon cuniculi infections in dogs: a case series. J Am Anim Hosp Assoc 45(5):225–231. https://doi.org/10.5326/0450225
Sobottka I et al (2001) Acute and long-term humoral immunity following active immunization of rabbits with inactivated spores of various Encephalitozoon species. Parasitol Res 87(1):1–6. https://doi.org/10.1007/s004360000297
Sokolova YY, Sakaguchi K, Paulsen DB (2016) Establishing a new species Encephalitozoon pogonae for the microsporidian parasite of inland bearded dragon Pogona vitticeps Ahl 1927 (Reptilia, Squamata, Agamidae). J Eukaryot Microbiol 63(4):524–535. https://doi.org/10.1111/jeu.12296
Suter C, Müller-Doblies UU, Hatt JM, Deplazes P (2001) Prevention and treatment of Encephalitozoon cuniculi infection in rabbits with fenbendazole. Vet Rec 148(15):478–480. https://doi.org/10.1136/vr.148.15.478
Szeredi L, Pospischil A, Dencsö L, Mathis A, Dobos-Kovács M (2007) A case of equine abortion caused by Encephalitozoon sp. Acta Vet Hung 55(4):525–532. https://doi.org/10.1556/AVet.55.2007.4.11
Talabani H, Sarfati C, Pillebout E, van Gool T, Derouin F, Menotti J (2010) Disseminated infection with a new genovar of Encephalitozoon cuniculi in a renal transplant recipient. J Clin Microbiol 48(7):2651–2653. https://doi.org/10.1128/jcm.02539-09
Tee KY et al (2011) Serological survey for antibodies to Encephalitozoon cuniculi in rabbits in Taiwan. Vet Parasitol 183(1–2):68–71. https://doi.org/10.1016/j.vetpar.2011.06.011
Thomas C, Finn M, Twigg L, Deplazes P, Thompson RC (1997) Microsporidia (Encephalitozoon cuniculi) in wild rabbits in Australia. Aust Vet J 75(11):808–810. https://doi.org/10.1111/j.1751-0813.1997.tb15658.x
Thurston-Enriquez JA, Watt P, Dowd SE, Enriquez R, Pepper IL, Gerba CP (2002) Detection of protozoan parasites and microsporidia in irrigation waters used for crop production. J Food Prot 65(2):378–382. https://doi.org/10.4315/0362-028x-65.2.378
Tsukada R, Osaka Y, Takano T, Sasaki M, Inose M, Ikadai H (2016) Serological survey of Encephalitozoon cuniculi infection in cats in Japan. J Vet Med Sci 78(10):1615–1617. https://doi.org/10.1292/jvms.15-0545
Wagnerová P et al (2012) Enterocytozoon bieneusi and Encephalitozoon cuniculi in horses kept under different management systems in the Czech Republic. Vet Parasitol 190(3–4):573–577. https://doi.org/10.1016/j.vetpar.2012.07.013
Wagnerová P, Sak B, Květoňová D, Maršálek M, Langrová I, Kváč M (2013) Humoral immune response and spreading of Encephalitozoon cuniculi infection in experimentally infected ponies. Vet Parasitol 197(1–2):1–6. https://doi.org/10.1016/j.vetpar.2013.05.007
Wang S et al (2018) Seroprevalence of Toxoplasma gondii and Encephalitozoon cuniculi among domestic rabbits in central China. Parasite 25:9. https://doi.org/10.1051/parasite/2018010
Wasson K, Peper RL (2000) Mammalian microsporidiosis. Vet Pathol 37(2):113–128. https://doi.org/10.1354/vp.37-2-113
Weber R et al (1997) Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection. N Engl J Med 336(7):474–478. https://doi.org/10.1056/nejm199702133360704
Xiao L, Li L, Visvesvara GS, Moura H, Didier ES, Lal AA (2001) Genotyping Encephalitozoon cuniculi by multilocus analyses of genes with repetitive sequences. J Clin Microbiol 39(6):2248–2253. https://doi.org/10.1128/JCM.39.6.2248-2253.2001
Xu Y, Weiss LM (2005) The microsporidian polar tube: a highly specialised invasion organelle. Int J Parasitol 35(9):941–953. https://doi.org/10.1016/j.ijpara.2005.04.003
Zietek J et al (2014) Diagnosis of the Encephalitozoon cuniculi infections in pet rabbits with neurological symptoms. Pol J Vet Sci 17(2):361–363. https://doi.org/10.2478/pjvs-2014-0050
Funding
This work was supported by the projects UIDB/CVT/00772/2020 and UIDB/00211/2020 funded by the Fundação para a Ciência e Tecnologia (FCT).
Author information
Authors and Affiliations
Contributions
Conceptualization: Tomás Rodrigues Magalhães, Filipe Fontes Pinto; formal analysis and investigation: Tomás Rodrigues Magalhães, Filipe Fontes Pinto; writing — original draft: Tomás Rodrigues Magalhães; writing — review and editing: Tomás Rodrigues Magalhães, Filipe Fontes Pinto, Felisbina Luisa Queiroga. Supervision: Felibina Luisa Queiroga.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have agreed to this final version of the manuscript and give their consent for its publication in the Parasitology Research journal.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Una Ryan
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Magalhães, T.R., Pinto, F.F. & Queiroga, F.L. A multidisciplinary review about Encephalitozoon cuniculi in a One Health perspective. Parasitol Res 121, 2463–2479 (2022). https://doi.org/10.1007/s00436-022-07562-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-022-07562-z