Abstract
Balamuthia mandrillaris is a free-living amoeba that lives in soil and water near human settlements. B. mandrillaris was first isolated from a mandrill baboon that died at the San Diego Zoo Wildlife Park in California in 1986, and the first human infection was reported in 1990. Although reported B. mandrillaris infections are often not properly characterized, it appears that B. mandrillaris invades the living body from the soil and water, either via a wound or the nasal cavity. Most confirmed infections have originated in South and North America. B. mandrillaris inhabits warm climates and is recognized as a pathogen in warm areas such as desert climates and tropical climates. B. mandrillaris has been isolated from environmental samples since 2000, most of which originated from warm areas such as step climates, tropical climates, and desert climates. However, B. mandrillaris may survive in diverse environments, although fewer granulomatous amebic encephalitis patients have been reported in colder Japanese and Northern European regions. In the present study, we conducted a survey of 13 soil samples in Aomori Prefecture located at the northernmost tip of Japan Honshu and successfully isolated one strain of B. mandrillaris from soil for the first time in Japan. In addition, B. mandrillaris gene was detected from several soils. This confirms that B. mandrillaris is capable of spreading to a wider climatic region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protozoan amoebae are classified according to their morphological and genetic features, and inhabit distinct niches in the environment, such as soil, seawater, and indoor dust (Schuster and Visvesvara 2004; Rodríguez-Zaragoza 2008). Among the many genera of free-living amoebae, only four have an association with human disease: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. These are termed amphizoic amoebae because they live both as parasites and in the natural environment. Free-living-amoebae can infect mammals, including humans, and cause granulomatous amebic encephalitis (GAE), skin amebiasis, and amebic keratitis (AK) (Szenasi et al. 1998; Schuster and Visvesvara 2004; Tsvetkova et al. 2004). Among these pathogenic amoebae, B. mandrillaris is the most recently discovered and is known to have high lethality, with over 90% of cases leading to death. B. mandrillaris was first discovered in a mandrill baboon (Papio sphinx) that died of encephalitis at the San Diego Zoo Wildlife Park in California in 1986. Subsequently, more than 200 human infections have been reported worldwide up to 2008 (Visvesvara et al. 1990; Siddiqui and Khan 2008).
Nine cases of Balamuthia infection have been confirmed in Japan (Table 1), and we confirmed an infection as recently as 2014 (Kenji 2010; Itoh et al. 2015). Among these domestic cases, healthy individuals without other immunological abnormalities have been identified, indicating a high risk of a generalized infection. In the present study, we isolated B. mandrillaris by cultivating soil collected in the Aomori Prefecture, which is at the northernmost tip of Honshu Island, in order to clarify the habitat of B. mandrillaris in the natural environment in Japan. Culturing B. mandrillaris from the natural environment is complicated and difficult (Niyyati et al. 2016), and we therefore attempted to detect the Balamuthia 16S rRNA gene in DNA extracted from soil samples.
Materials and methods
Collection of soil samples
Surface soil was collected at a depth of about 10 cm after removing leaf debris. Ten samples were collected in Hachinohe city located on the southern part of the Pacific side, and three samples in Hirosaki located on the inland western part of Aomori Prefecture (Fig. 1). Soil was collected in general households, agricultural lands, university premises, and a shrine (Table 2).
The collected soil was cultured prior to extraction of DNA for Balamuthia-specific PCR. Soil samples were stored in a refrigerator at 4 °C and used for culture and DNA extraction within 3 months of collection.
Isolation of amoebae by soil culture
Acanthamoeba type II (MK strain) from our laboratory collection was heat treated (heat-treated Acanthamoeba MK: HTAM) and spread on 1.5% non-nutrient (NN) agar for cultivation. Acanthamoeba MK strain used for preparation of HTAM was cultured in PYG medium to a confluent state. Thereafter, Acanthamoeba MK strain washed twice with pH 7.2 phosphate-buffered saline (PBS(−)), adjusted to 3000/μl and heat treated by autoclaving at 121 °C for 15 min. Heat-treated Acanthamoeba MK strain was cultured in PYG medium and confirmed dead. Soil (10 g) was suspended in 10 ml of KCM medium (7 mg/l KCl, 8 mg/l CaCl2, and 8 mg/l MgSO4•7H2O), and large soil particles were removed using gauze filtration. The filtrate was centrifuged at 800g for 5 min to obtain sediment. The sediment was applied at the center of a petri dish containing 1.5% NN agar medium and cultured at 30 °C for 10 days. Each culture was inspected once daily. When large dendritic amoebae were observed, the colony was excised and transplanted on fresh agar. The large amoebae were subsequently cultured in liquid Balamuthia medium BM-3 (Schuster and Visvesvara 1996) and soil solution (SS) medium.
SS medium was prepared by adding 50 g of soil to 1000 ml of physiological saline. The soil suspension was centrifuged at 800g for 5 min, and the supernatant was recovered and sterilized by filter sterilization with a 0.22-μm filter (Sartolab, Germany) as the SS medium. The soil type used to prepare SS medium has previously been used to ascertain the habitat of large amoebae.
DNA extraction from soil
Soil (10 g) was weighed in a conical tube, and 4 g of 350 μmΦ glass beads and 400 mg of skim milk were added. This was followed by the addition of 4 ml of sodium dodecyl sulfate (SDS) lysis buffer [0.5 M Tris HCl(pH 8.0), 0.1 M NaCl, 10% SDS, filter sterilized after creation] and 10 ml of pH 8.0 PBS (−), and the mixture was then vigorously stirred at 1300g for 15 min. After stirring, the mixture was heated at 60 °C for 5 min and then stirred at 1300g for 15 min. The soil mixture was centrifuged at 2300g for 10 min, and the supernatant was recovered. An equal volume of phenol:chloroform:isoamyl alcohol (25:24:1, PCI; NIPPON GENE, Japan) was added to the supernatant, and the mixture was vigorously stirred and then centrifuged at 9000g for 20 min. After recovering the supernatant, isopropanol and 3 M sodium citrate were added, and the mixture was incubated at − 30 °C for 30 min, and then centrifuged at 13,000g for 20 min to obtain the sediment. The sediment was dissolved in 600 μl of distilled water and finally purified using an Isoil DNA extraction kit (NIPPON GENE, Japan).
DNA extraction of isolated amoeba
Large amoeba subcultured on 1.5% NN agar were recovered in KCM medium and then centrifuged at 800g for 5 min for DNA purification using a QIAamp DNA Mini Kit (QIAGEN, USA).
Balamuthia-specific polymerase chain reaction and sequencing
The purified DNA was adjusted to 10~200 ng/μl, and PCR was carried out using with Buffer II (Thermo Fisher Scientific, USA). The PCR primer was 5’Balspec 16S (5′-CGCATGTATGAAAGAAGACCA-3′) and Balspec 16Sr 610 (5′-CCCCTTTTTAACTCTAGTCATATAGT-3′), with an expected product of 230 bp (Itoh et al. 2015). PCR conditions were 35 cycles of thermal denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 45 s. After PCR, bands were confirmed on a 1.2% agarose gel by electrophoresis, and then excised for sequencing by TA cloning using a Mighty TA-cloning kit (Takara Bio, Japan). The gene sequence was decoded using a model 3130 genetic analyzer (Applied Biosystems, USA) and analyzed using MEGA Ver. 7 software.
The 5′Balspec16S (5′-CGCATGTATGAAGAAGACCA-3′) and 3′Balspec 16S (5′-TTACCTATATAATTGTCGATACCA-3′) primer were also used for AHB strain sequencing, with an expected product of 1075 bp (Tavares et al. 2006).
Results
Separation of culture and B. mandrillaris
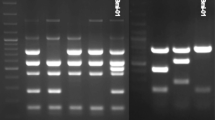
Large dendritic amoebae were present in samples cultured from several sites after 5 days of culturing (Fig. 2(A)). Six types of dendritic large amoebae were isolated from six of 13 soil samples. The DNA extracted from the “amoeba No.1” sample, obtained from a horse farm in Hachinohe, was positive for Balamuthia-specific DNA (Fig. 3). This PCR product was used for sequencing after TA cloning and had 99% homology with the Balamuthia 16S rRNA sequence. The isolated amoeba was designated as the “AHB” strain (Aomori-Hachinohe-Balamuthia Strain). A longer portion (1075 bp) of the 16S gene was used to confirm the identity of this strain following PCR and TA cloning (Fig. 3), and the resulting product again shared 99% homology (Table 3) with the B. mandrillaris 16S gene (accession number: LC348995).
Balamuthia-specific-PCR for isolated amoebae and soil DNA. Lane (1) 100-bp ladder (Nippon Gene, Japan), lane (2) negative control, primer: 5′Balspec 16S and Balspec 16Sr 610, lane (3) sample of AHB strain, Primer: 5′Balspec 16S and Balspec 16Sr 610, lane (4) negative control, primer: 5′Balspec 16S and 3′Balspec 16S, lane (5) sample of AHB strain, primer: 5′Balspec 16S and 3′Balspec 16S, lanes (6, 7, 8) positive sample of soil DNA, primer: 5′Balspec 16S and Balspec 16Sr 610
The nutritional type of the AHB strain was 15 to 45 μm in size and the cyst was 5 to 15 μm, which is similar to the morphology of B. mandrillaris in the literature (Schuster et al. 2003; Niyyati et al. 2009) (Fig. 2(B-1), (B-2), (B-3)).
In order to subculture the six large amoebae isolated from the soil samples, we switched from plate culture to BM-3 liquid culture, which is a suitable medium for culturing B. mandrillaris (Schuster and Visvesvara 1996). However, the amoebae did not survive in liquid BM-3 culture, and we therefore created a new simplified medium. We designed a medium containing soil, as we hypothesized that it would best support amoebae isolated from the soil environment. To test the new medium, we cultivated a laboratory strain of B. mandrillaris of environmental origin. The transplanted amoebae temporarily differentiated and proliferated, but gradually weakened and died without forming cysts.
Balamuthia-specific PCR using soil DNA
In the present study, 13 soil samples were cultivated to obtain six large amoebae cultures. These were separated from soil of sampling place No. 1, 4, 5, 7, 10, and 13. Among these, only one clone was successfully obtained (AHB strain) from soil of sampling place No. 1. For this reason, we extracted DNA from 13 soil samples and used the DNA to confirm the presence of Balamuthia by Balamuthia-specific-PCR. As a result, Balamuthia-specific PCR amplification products in three soil samples (No. 7, 8, 10) were confirmed (Fig. 3). Following sequencing and analysis of these PCR products, we found that these putative Balamuthia strains shared 99% homology with previously reported B. mandrillaris. The accession numbers of these three PCR products are LC349292, LC349293, and LC349294.
Discussion
B. mandrillaris was first discovered in 1986 from cerebral necropsy of a mandrill baboon (P. sphinx) that died of neurological disease at the San Diego Wildlife Park in California (Rodríguez-Zaragoza 2008), and is thus a relatively recently discovered pathogen. Since then, more than 200 cases of meningoencephalitis caused by B. mandrillaris had been reported worldwide by 2008 (Visvesvara et al. 1990; Siddiqui and Khan 2008). Many of the infected people are Hispanic with agriculture-related occupations, which involve contact with the soil, and may have provided the opportunity for infection (Schuster and Visvesvara 2004; Jackson et al. 2014). Consistent with this, B. mandrillaris has been found in environmental samples since the 2000s. To date, eight environmental cases have been confirmed, comprising two cases isolated from the soil in flowerpots in the USA (Schuster et al. 2003; Yagi et al. 2005), two from Iran (one from soil and one from city dust) (Niyyati et al. 2009, 2016), one from well water in Guinea-Bissau (Baquero et al. 2014), one from Peruvian soil (Cabello-Vílchez et al. 2014), one from water in Mexico (Lares-Jiménez et al. 2014), and one from a mud bath in Jamaica (Todd et al. 2015). These cases originated in regions with tropical climates, desert climates, and step climates. Similarly, the cases of human infection are concentrated in warm climates (Schuster and Visvesvara 2004). However, in the present study, we isolated B. mandrillaris from a heavy snow area in Japan, suggesting that this pathogen can survive in a wider range of environments around the world.
In the present study, we did not characterize the pathogenicity of the AHB. Although we considered it necessary to investigate the pathogenicity of the isolated strain, and attempted mass culture using the devised SS liquid medium, the AHB Strain could not be successfully cultured in this medium. For this reason, although SS medium was specifically developed for mass culturing soil-derived B. mandrillaris, it did not enable long-term cultivation of this strain. Cultivation of environmental strains may not be successful using currently available culture-based methods. It is necessary to develop new methods, which are suitable for mass culture of environmental isolates.
Conclusions
The habitat of B. mandrillaris may extend to a wider area than previously expected. B. mandrillaris infection has a strong association with immune dysfunction, and so is likely to occur in infants, middle-aged, and elderly people, and those with underlying diseases such as HIV (Perez and Bush 2007; Schuster and Visvesvara 2004). In Japan, where the proportion of elderly people is increasing, such infections may become more prevalent in the future. While B. mandrillaris is currently classified as an infectious disease of warm climates like tropical climates and tropical climates, we have shown that it can be isolated in cold regions with heavy snow and must therefore re-assess the risk profile of this infectious amoeba in such regions.
References
Alexander L G, Kevin Messacar, Thelma Dunnebacke, Samia N Naccache, Scot Federman, Jerome Bouquet, David Mirsky, Yosuke Nomura, Shigeo Yagi, Carol Glaser, Michael Vollmer, Craig A Press, Bette K Klenschmidt-DeMasters, Samuel R Dominguez, Charles Y Chiu (2015) Clinical metagenomic identification of Balamuthia mandrillaris encephalitis and assembly of the draft genome: the continuing case for reference genome sequencing. Genome Med 7: 113 https://doi.org/10.1186/s13073-015-0235-2
Bando Y, Takahashi T, Uehara H, Kagegi T, Nagahiro S, Izumi K (2012) Autopsy case of amebic granulomatous meningoencephalitis caused by Balamuthia mandrillaris in Japan. Pathol Int 62(6):418–423. https://doi.org/10.1111/j.1440-1827.2012.02816.x
Baquero RA, Reyes-Batlle M, Nicola GG, Martın-Navarro CM, Lopez-Arencibia A, Guillermo Esteban J, Valladares B, Martınez-Carretero E, Pinero JE, Lorenzo-Morales J (2014) Presence of potentially pathogenic free-living amoebae strains from well water samples in Guinea-Bissau. Pathog Glob Health 108(4):206–211
Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA (2003) Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg 68(1):65–69
Cabello-Vílchez AM, Reyes-Batlle M, Montalbán-Sandoval E, Martín-Navarro CM, López-Arencibia AE-L, Guerra HR, Gotuzzo E, Piñero J, Maciver S, Martínez-Carretero E, Valladares B, Lorenzo-Morales J (2014) The isolation of Balamuthia mandrillaris from environmental sources from Peru. Parasitol Res 113(7):2509–2513. https://doi.org/10.1007/s00436-014-3900-2
Dunnebacke TH, Schuster FL, Yagi S, Booton GC (2004) Balamuthia mandrillaris from soil samples. Microbiology 150(9):2837–2842
Hayashi T, Yamanaka E, Nabeshima K, Sumiyoshi A, Meiwa Y (1996) An autopsy case of primary amoebic meningoencephalitis. Proc Jpn Soc Pathol 85:172 (in Japanese)
Hayashi R, Koide T, Yamada M, Nagai H, Takahashi H (1997) An autopsy case of amebic granulomatous encephalitis. Neuropathology 17:203 (in Japanese)
Itoh K, Yagita K, Nozaki T, Katano H, Hasegawa H, Matsuo K, Hosokawa Y, Tando S, Fushiki S (2015) An autopsy case of Balamuthia mandrillaris amoebic encephalitis, a rare emerging infectious disease, with a brief review of the cases reported in Japan. Neuropathology 35(1):64–69. https://doi.org/10.1111/neup.12151
Jackson BR, Kucerova Z, Roy SL, Aguirre G, Weiss J, Sriram R, Yoder J, Foelber R, Baty S, Derado G, Stramer SL, Winkelman VVG (2014) Serologic survey for exposure following fatal Balamuthia mandrillaris infection. Parasitol Res 113(4):1305–1311. 2509-2513. https://doi.org/10.1007/s00436-014-3900-2
Kato H, Mitake S, Yuasa H, Ayashi H, Hara T, Matsukawa N (2013) Successful treatment of granulomatous amoebic encephalitis with combination antimicrobial therapy. Intern Med 52(17):1977–1981
Kenji Y (2010) Amebic meningoencephalitis IASR. 31(11):334–335. < http://idsc.nih.go.jp/iasr/31/369/kj3695.html>2017/2/2
Kinde H, Visvesvara GS, Barr BC, Nordhausen RW, Chiu PH (1998) Amebic meningoencephalitis caused by Balamuthia mandrillaris (leptomyxid ameba) in a horse. J Vet Diagn Investig 10(4):378–381. https://doi.org/10.1177/104063879801000416
Lares-Jiménez LF, Booton GC, Lares-Villa F, Velázquez-Contreras CA, Fuerst PA (2014) Genetic analysis among environmental strains of Balamuthia mandrillaris recovered from an artificial lagoon and from soil in Sonora, Mexico. Exp Parasitol 145(S):57–61
Monobe Y, Morisada Y, Shirabe T (1991) An autopsy case of primary amoebic meningoencephalitis due to free living ameba (Leptomyxid). Neuropathology 11:357 (in Japanese)
Nakamura T, Kobayashi M, Wada H, Tsunoda Y, Akai K, Omata K (1979) An autopsy case of primary amoebic meningoencephalitis. Adv Neurol Sci 23:500–509 (in Japanese with English abstract)
Niyyati M, Lorenzo-Morales J, Rezaeian M, Martin-Navarro CM, Haghi AM, MacIver SK, Valladares B (2009) Isolation of Balamuthia mandrillaris from urban dust, free of known infectious involvement. Parasitol Res 106(1):279–281
Niyyati M, Karamati SA, Lorenzo Morales J, Lasjerdi Z (2016) Isolation of Balamuthia mandrillaris from soil samples in North-Western Iran. Parasitol Res 115(2):541–545
Perez MT, Bush LM (2007) Balamuthia mandrillaris amebic encephalitis. Curr Infect Dis Rep 9: 323–328
Rodríguez-Zaragoza S (2008) Ecology of free-living amoebae. J Crit Rev Microbiol 25:225–241
Schuster FL, Visvesvara GS (1996) Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amebic meningoencephalitis in humans and other animals. J Clin Microbiol 34(2):385–388
Schuster FL, Visvesvara GS (2004) Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34(9):1001–1027
Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, Vugia D, Bakardjiev A, Azimi P, Maddux-Gonzalez M, Julio Martinez A, Visvesvara GS (2003) Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol 41(7):3175–3180
Siddiqui R, Khan NA (2008) Balamuthia amoebic encephalitis: an emerging disease with fatal consequences. Microb Pathog 44(2):89–97
Sugita Y, Fujii T, Hayashi I, Aoki T, Yokoyama T, Morimatsu M, Fukuma T, Takamiya Y (1999) Primary amebic meningoencephalitis due to Naegleria fowleri: an autopsy case in Japan. Pathol Int 49(5):468–470
Szenasi Z, Endo T, Yagita K, Nagy E (1998) Isolation, identification and increasing importance of “free-living” amoebae causing human disease. J Med Microbiol 47(1):15–16
Tavares M, Da Costa JMC, Carpenter SS, Santos LA, Afonso C, Aguiar Á, Visvesvara GS (2006) Diagnosis of first case of Balamuthia amoebic encephalitis in Portugal by immunofluorescence and PCR. J Clin Microbiol 44(7):2660–2663
Todd CD, Reyes-Batlle M, Piñero JE, Martínez-Carretero E, Valladares B, Lindo JF, Lorenzo-Morales J (2015) Balamuthia mandrillaris therapeutic mud bath in Jamaica. Epidemiol Infect 143: 2245–2248
Tsvetkova N, Schild M, Panaiotov S, Kurdova-Mintcheva R, Gottstein B, Walochnik J, Müller N (2004) The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol Res 92(5):405–413
Uenohara K, Okudaira K, Yajima M (1987) A case of granulomatous meningo-encephalitis by Acanthamoeba. Niigata Igaku 101:655 (in Japanese)
Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, Anderson M (1990) Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol 28(12):2750–2756
Yagi S, Booton G, Visvesvara G, Schuster F (2005) Detection of Balamuthia mandrillaris 16S rRNA gene DNA in clinical specimens by PCR. J Clin Microbiol 43(7):3192–3197
Yamasaki K, Sugimoto T, Futami M, Moriyama T, Uehara H, Takeshima H, Moriguchi S, Marutsuka K, Asada Y (2011) Granulomatous amoebic encephalitis caused by Balamuthia mandrillaris. Neurol Med Chir 51(9):667–670
Funding
This work was supported by the JSPS KAKENHI Grant-in-Aid for Young Scientists (B) Numbers JP15K19240.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yamanouchi, K., Arima, H., Sakamoto, Y. et al. First report of the isolation of Balamuthia mandrillaris in the northern region of Japan. Parasitol Res 117, 2895–2900 (2018). https://doi.org/10.1007/s00436-018-5980-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-5980-x