Abstract
The poultry red mite (PRM) Dermanyssus gallinae causes high economic losses and is among the most important parasites in poultry farming worldwide. Different chemical, physical, and biological strategies try to control the expansion of PRM. However, effective solutions to this problem still have to be found. Here, we present a method for the development of an immunological control strategy, based on the identification of mite protein antigens which elicit antibodies with anti-mite activity in the immunized chicken. Hens were immunized with different PRM protein extracts formulated with two different adjuvants, and IgY-antibodies were isolated from the eggs. A PRM in vitro feeding assay which used chicken blood spiked with these IgY-preparations was used to detect antibodies which caused PRM mortality. In vitro feeding of mites with IgY isolated from hens immunized with PRM extract formulated with one of the adjuvants showed a statistically significant increase in the mortality as compared to control mites. After the separation of total PRM extracts in two-dimensional gels, several protein spots were recognized by such IgY preparations. Ten protein spots were subjected to mass spectrometry (MS/MS) for the identification of the corresponding proteins. Complete protein sequences were deduced from genomic and transcriptomic assemblies derived from high throughput sequencing of total PRM DNA and RNA. The results may contribute to the development of an immunological control strategy of D. gallinae.
Similar content being viewed by others
Introduction
The blood-sucking ectoparasite Dermanyssus gallinae (De Geer 1778), also known as the poultry red mite (PRM), usually infests domestic birds but can also feed on other animals and also humans, when the main host is absent (Brockis 1980). PRM can bite through the skin of the host to feed on blood, causing itching, irritation, and stress to the host and may even cause death in commercial poultry farms due to anemia (Kirkwood 1967; Schicht et al. 2013). PRM are also potential vectors for several pathogens, such as viruses and bacteria (Moro et al. 2009). Due to a significant impact on health and egg production, PRM causes high economic losses in poultry industry worldwide and is recognized as a vast economic, welfare, and epidemiological problem for both birds and humans (Sparagano et al. 2009). In Europe alone, the annual costs for the poultry industry caused by PRM were estimated to be about 130 million euro in 2005 (van Emous 2005). Therefore, different methods are being used to try to control PRM infestations (Schicht et al. 2014). Some strategies involve frequent cleaning of the poultry farms and application of desiccant powders (Kilpinen and Steenberg 2009), repellents derived from plant oils (Carroll 1994; George et al. 2009; Birkett et al. 2011), or the treatment with acaricidal agents or traps (Chirico and Tauson 2002). Other strategies focusing on biological methods have been tested, such as insect growth regulators, infection of PRM with pathogenic bacteria (Chauve 1998), fungi (Steenberg and Kilpinen 2014), or the use of predatory mites (Lesna et al. 2012). However, none of these strategies has proven largely successful in eradicating the pest from poultry farms, and there is a high demand for innovative approaches for the control of PRM.
An alternative to existing strategies to combat PRM is based on vaccination (Sparagano 2009). The underlying principle consists of antibodies which are generated in the host and are taken up by the parasite during the blood meal. If these antibodies are directed against an antigen present inside of the parasite (Sauer et al. 1994), they might have a negative effect onto their target tissue, thereby causing harm or even death of the parasite. Vaccines are non-toxic, do not cause problems with residual chemicals, and resistance is unlikely to emerge. The first such animal vaccine against a tick (Boophilus microplus) was licensed in 2000 (Jonsson et al. 2000) and consists of the protein Bm86. This antigen was identified via a laborious procedure involving multiple rounds of immunizations of cows with biochemical tick extract fractions (Willadsen and Kemp 1988; Willadsen et al. 1989). The tick protein Bm86 was also tested for a potential protection of hens from PRM, but results were negative, and a direct homolog of Bm86 could not be identified in D. gallinae (Harrington et al. 2009a). On the other hand, immunization of hens with PRM protein extracts led to protection against PRM, as by applying in vitro PRM feeding of specific antibody preparations elevated PRM mortalities compared to control preparations were observed (McDevitt et al. 2006; Harrington et al. 2009b; Wright et al. 2009). In addition, histamine-releasing factor and cathepsin D were investigated as potential vaccine candidates through gene-knockdown approaches (Kamau et al. 2013).
Here, a strategy is presented to identify potentially protective antigens in PRM extracts via combining biochemical fractionations, in vitro feeding of antibodies to PRM and proteomic techniques. By using this approach, a number of proteins are identified which have the potential to serve as candidate antigens for a vaccine against D. gallinae.
Material and methods
D. gallinae protein isolation
PRM were collected from egg production poultry farms in Thuringia, Germany. The storage conditions and protein isolation of PRM were modified from previously used protocols (McDevitt et al. 2006; Harrington et al. 2009b; Wright et al. 2009). The mites were stored in a 75 cm2 CellStar tissue culture flask (Greiner Bio-One) with a filter cap. Before PRM protein isolation started, the mites had starved in the dark for 3–7 days at room temperature (RT), to allow the digestion of chicken blood. Then, these mites were transferred to a refrigerator and maintained at 5 °C for 3–4 weeks. Mites (eggs, larvae, starved nymphs, adult female and males) were subjected to homogenization and sonication on ice in different buffers (see below). For the isolation of PRM soluble proteins, as previously described (Harrington et al. 2009b), about 500 mites were homogenized with a micropestle in microtubes between the centrifugation step and sonication on ice in 0.5 ml phosphate-buffered saline (PBS), pH 7.4, containing 1× EDTA-free protease inhibitor (Roche). These steps (homogenization, sonication, and centrifugation) were repeated three times before the homogenized mites were incubated for 20 min at 4 °C and centrifuged at 24,000×g for 20 min at 4 °C. The supernatant was retained, and the pellet was homogenized as described before, but with a buffer containing 8 M urea (to retrieve insoluble proteins) in PBS. The homogenate was incubated for 90 min at 37 °C before centrifugation at 24,000×g for 20 min at RT, and the supernatant was used for immunization. Both soluble and insoluble extract fractions were administered in separate syringes on separate locations. Two hundred-microgram protein (100-μg soluble and 100-μg insoluble proteins, as determined by Bradford protein measurements) was used per dose per animal, based on previous work (Harrington et al. 2009b).
The isolation of PRM proteins for the analysis on 2D SDS PAGE was similar to the protein isolation protocol used before, except the usage of an isolation buffer containing 7 M urea, 2 M thiourea, 2 % v/v CHAPS, and traces of bromophenol blue (0.002 % v/v).
2D SDS PAGE and Western blots
To identify individual mite proteins, 2D SDS PAGE analysis and Western blots were performed for PRM. First, PRM proteins were separated by 2D SDS PAGE using an Ettan IPGphor III IEF System (GE Healthcare) following the manufacturer’s instructions and based on a protocol described previously (Pohler et al. 2012). Briefly, at the beginning of the passive rehydration, the thawing PRM extract was supplemented with rehydration buffer (7 M urea, 2 M thiourea, 2 % v/v CHAPS, 0.002 % v/v bromophenol blue) containing 1.3 % v/v fresh added Ampholine (GE Healthcare). Thereafter, for the passive rehydration of 7-cm Immobiline DryStrip pH3-10 NL (GE Healthcare, Sweden), 200-μg PRM protein extract diluted in 100 μl of rehydration buffer was used. After 16 hours passive rehydration at RT, the isoelectric focusing (IEF) did run for 3.5 h on an Ettan IPGphor III IEF System (GE Healthcare). The running conditions were 300 V for 60 min followed by linear gradients of 1 kV for 30 min, 5 kV for 90 min, and 5 kV for 30 min. Succeeding focusing, IPG strips were incubated with equilibration buffers as described before (Pohler et al. 2012). In the second equilibration buffer, 2.5 % v/v iodoacetamide was added to prevent re-oxidation by atmospheric oxygen (Sechi and Chait 1998; Herbert et al. 2001). Finally, the second dimension was performed on 12 % SDS gels, which were stained with Coomassie Brilliant Blue (Fluka) or used for Western blotting.
Western blots: PRM proteins were separated through 2D SDS PAGE before transfer onto nitrocellulose membranes overnight at 4 °C. Antibodies were isolated from eggs collected from immunized hens following the manufacturer’s instructions of the Pierce Chicken IgY Purification Kit (Thermo scientific). IgY were diluted 1:50 with 5 % v/v milk (skimmed milk powder AppliChem) in PBS-Tween (0.1 v/v Tween 20) and 300 μg IgY (IgY preparations used for the in vitro feeding, see below) was incubated with the membrane for 2 h. Next, the membrane was incubated for another 2 h with the peroxidase conjugated rabbit anti-chicken IgY antibody (Sigma) diluted 1:20,000. After each incubation step, the membrane was washed three times with PBS-Tween. The blots were developed using ECL Western Blotting Substrates (Thermo Scientific).
The stained 2D SDS gels and the Western blot films were digitized using an Image Scanner III (GE Healthcare). Subsequent computer-assisted image analysis of the PRM 2D gels and Western blot images were processed using the Delta2D 3.6 software (DECOCON, Germany) according to the manufacturer’s instructions.
Immunization of hens and adjuvant tests
Ethical approval for animal experiments was obtained under Reg.-Nr.: TVV 40/10 from Landesdirektion Leipzig, Germany. Sixteen Lohmann Brown hens (obtained from commercial poultry breeders in Saxony, Germany) at 18 weeks of age were acclimatized for a minimum of 1 week in rooms of the Clinic for Birds and Reptiles, Leipzig University. The pre-experimental and experimental phases were conducted in the same room. Water was provided ad libitum, as well as commercial non-medicated breeding hen feed. Lighting was provided on a cycle of 12-h light and 12-h dark, and the hens had the opportunity to perch on during the night.
The performance and health of hens were controlled by the staff of the Clinic for Birds and Reptiles, Leipzig University by veterinary daily observation. Any hen which showed signs of illness was examined closely. During the vaccination process, a short physical examination was performed.
All animals were negative in an ELISA containing PRM antigens before immunization (data not shown). For the production of antibodies against mite proteins, hens were immunized subcutaneously with total PRM extract (see above). Hens were vaccinated three times, at day 0, 28, and 56. Two different adjuvants, Freund’s complete and incomplete adjuvant (Sigma) and Montanide ISA 70 VG (Seppic) were tested. Controls were immunized with PBS and adjuvant only. Freund’s adjuvant (F) was administered in the ratio of 1:1 to provide a total volume of 300 μl vaccine per hen/immunization, and Montanide ISA 70 VG adjuvant (M) was administered in the ratio of 7:3 to provide a total volume of 1 ml vaccine per hen/immunization as recommended by the manufacturers.
The 16 hens were randomly allocated to one of four groups (four hens/group): Two control groups (F and M adjuvants only) and two groups immunized with PRM proteins (200 μg per dose) and the adjuvants, respectively.
Chicken blood (4 ml) was collected from the vena ulnaris into a lithium heparin tube (Sarstedt, Germany) before each immunization and also 4 weeks after the third immunization for the analysis of anti-PRM antibodies using an ELISA-test (Chabierski et al. 2013). Briefly, Nunc polysorb plates (Thermo Scientific) were coated overnight with 100 ng per well of total PRM extract before sera from immunized hens were diluted 1:100 and used as first antibody. Bound IgY were detected with a rabbit anti-chicken antibody (1:20 000, see above). ELISA-plates were developed with TMB-substrate, (BioLegend, Germany). The values of the optical density represent the mean of triplicate measurements detected at 450 nm (520 nm as reference wavelength) in an ELISA Reader (Infiniti M200, Tecan).
In vitro feeding of PRM
A PRM in vitro feeding system based on previously published protocols (McDevitt et al. 2006; Harrington et al. 2009b; Wright et al. 2009) was performed. Briefly, skins from frozen 1-day-old chicks (obtained from the Clinic for Birds and Reptiles, Leipzig University) were plucked, washed and stored at −20 °C for 1–2 weeks before use. The blood reservoir was constructed from an inverted 10-ml pipette tip (Gilson) covered with a 2-cm2 strip of the chick skin left at RT for at least 10 min. The outer surface of the chick skin was exposed to the mites in a glass tube (DURAN), and the internal surface of the chick skin was in contact with chicken blood as previously described (McDevitt et al. 2006). Two hundred fifty-microliter fresh heparinized chicken blood from birds without detectable RVM-antibodies (data not shown) used within 6 h after collection was spiked with antibodies (750 μg in 50 μL) isolated from chicken eggs (pooled samples from four eggs per group) collected 2–4 weeks after the third immunization. In each glass tube, a filter paper (9 mm × 95 mm) was placed, and 10–20 PRM (adult females) were incubated in the dark with the blood reservoir 16 h at 35 °C ± 3 °C and relative humidity of 70–80 %. After mites were engorged, they were transferred from 35 °C to RT and were monitored 1 week after the feeding for mortality. During this period, the mortality of 80–130 PRM was recorded per experiment, divided into triplicates for each test. If mites were unresponsive to a stimulus with a needle according to (McDevitt et al. 2006), they were defined as dead.
Proteomics
After PRM protein separation through two-dimensional gel electrophoresis, protein spots were selected in four independent repetitions of Western blots from the Montanide ISA 70 VG group. Ten protein spots were cut from the 2D gel and the proteins were partially sequenced by Mass spectrometry (MS/MS) in a 4700 Proteomics Analyzer (AB Sciex). To obtain a reference genome and transcriptome to deduce protein sequences, total DNA and RNA were isolated from PRM (eggs, larvae, starved nymphs, adult female and males) according to the manufacturer’s instructions (TRI Reagent, Sigma), and 1–2 μg each (DNA and DNase-treated RNA) was applied for the library generation using the TrueSeq RNA kit v2 as well as the TrueSeq DNA kit (Illumina). Paired-end sequencing was performed with 2× 100 bp using the HiSeq 2000 resulting in 183 mio. DNA reads and 354 mio. RNA reads providing 18.3 GB and 35.4 GB, respectively.
Subsequently, the bioinformatics analysis was performed as follows: sequencing adapters, indices, and index adapters were removed with the software Cutadapt version 1.3 (Martin 2011). The revised RNA reads were then assembled to sets of contigs, representing partial or full transcripts, with Trinity version r20140413p1 (Grabherr et al. 2011). In the next step, the proteins coded by this set of contigs were predicted in-silico with the software TransDecoder (Haas et al. 2013). The tandem of MS and MS/MS data was processed as follows: Peaks in MS/MS spectra were detected using the statistical software R version 3.1 (R Core Team 2014) and additional R packages: MALDIquant 1.10 (Gibb and Strimmer 2012), MALDIquantForeign 0.8 (Gibb 2014), and plyr 1.81 (Wickham 2011). Finally, MS/MS spectra peaks were matched with predicted proteins using the software X!Tandem version 13-09-1 (Craig and Beavis 2004).
Statistical analyses
The statistical analysis of the ELISAs was carried out using SigmaPlot (Systat Software, San Jose, CA, USA) for the Mann-Whitney Rank Sum Tests. For the in vitro feeding studies, the mortality rates of mites in each treatment group after 1 week were carried out using R software version 3.2.2 (R Core Team 2015) with R packages lme4 (Bates et al. 2015) and multcomp (Hothorn et al. 2008). The mortality was modeled with a generalized linear mixed effects model with treatment groups and adjuvant groups as two fixed effects and feeding assays and observation level data as random effects. For the mortality, a binomial distribution with a logit link function was used. The statistical significance was declared at p < 0.05. The proteomics results were analyzed and clustered with statistics software R (function “hclust” using a complete-linkage approach) as well as the program stretcher (for alignment and similarity assessment) of the software suite EMBOSS version 6.6.0.0 (Rice et al. 2000).
Results
Immunization with PRM extracts formulated with different adjuvants
Several adjuvants have been used with PRM immunizations before (Arkle et al. 2008; Harrington et al. 2009b; Wright et al. 2009), and the results differed. Therefore, we decided to use both Freund’s adjuvant (F) and Montanide ISA 70 VG (M) to determine the most efficient conditions for the production of antibodies against PRM (Fig. 1). To monitor the appearance of PRM antibodies, sera were collected from hens before each immunization and 4 weeks after the third immunization. The results indicate specific humoral immune responses against PRM proteins upon immunization (Fig. 1). The IgY-titer was significantly higher (Mann-Whitney Rank Sum Test, p < 0.03) in hens immunized with PRM-M and PRM-F extracts as compared to hens from the corresponding control group immunized with the same adjuvant. In addition, antibody levels were higher in the PRM-M group as compared to the PRM-F group (Fig. 1) indicating different effects of the adjuvants on the immune response.
Detection of specific antibody responses against total PRM extracts by ELISA using sera from 16 hens 4 weeks after the last immunization with PRM total extracts. Two different adjuvants (F Freund’s adjuvant, M Montanide ISA 70 VG adjuvant) were tested. Control animals were immunized with PBS and adjuvant only. Mean values of three independent experiments (performed in triplicate) are shown, and error bars represent the standard deviation. Statistical analysis to evaluate the difference between the antibody response was performed by using a Mann-Whitney Rank Sum Test. Asterisks indicate statistically significant differences (p < 0.03) between the anti-mite IgY production in hens immunized with PRM extracts and the corresponding control hens. Optical Density (O.D.) signals were read out at 450 nm with background reduction at 520 nm
In vitro feeding with PRM IgY-Fractions
IgY was isolated from eggs of immunized hens. To detect antibodies which may lead to mortality of mites upon ingestion, PRM in vitro feeding assays were performed. Mites were monitored for 1 week after the in vitro feeding, and the mortality rates between the groups were analyzed. The results indicate (Table 1 and Fig. 2) that the IgY derived from hens immunized with PRM-F extracts only (PRM IgY-F) led to a marginal increase in PRM mortality. In contrast, the mortality of the PRM fed with IgY isolated from hens immunized with PRM-M extract (58.3 %) was higher than the mortality of mites fed with IgY isolated from control hens (22.5 %), and the difference was statistically significant (p < 0.01) as determined by a generalized linear mixed effects model.
PRM mortality (in %) after Dermanyssus gallinae in vitro feeding with fresh heparinized chicken blood spiked with 750 μg antibodies. Mortality was monitored for 1 week in three independent experiments (performed in triplicate). Controls are PRM fed with IgY isolated from hens immunized without PBS and adjuvant only. PRM IgY-F was isolated from hens immunized with PRM extract and Freund’s adjuvant. PRM IgY-M was isolated from hens immunized with PRM extract and Montanide ISA 70 VG adjuvant. Error bars represent the standard deviation. Statistical analysis to evaluate the difference between the mortality was performed by using a generalized linear mixed effects model. The difference between the mortality of mites fed with control IgY-M and mites fed with PRM IgY-M was 35.8 % and statistically significant (p < 0.01)
Therefore, this increase in mortality of 35.8 % (Table 1 and Fig. 2) proposes the presence of anti-mite activity displayed by the antibodies isolated from hens immunized with PRM-M extracts.
Analysis of the protein pattern recognized by antibodies against the PRM-M fraction
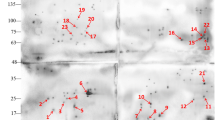
To visualize individual protein antigens which are recognized by IgY preparations leading to PRM mortality, total PRM extracts were analyzed by 2D SDS PAGE and Western blots. As can be seen in Fig. 3, the immune detection indicate highly specific antibody production against PRM proteins by the hens immunized with PRM-M and PRM-F, whereas almost no proteins were stained when IgY from control-immunized animals were used. Several protein spots were uniformly recognized by IgY from the PRM-M and PRM-F immunized hens. Because mite mortality was significantly increased only with IgY from PRM-M-immunized chicken (Table 1 and Fig. 2), 2D gel protein spots exclusively recognized by this IgY-preparation are considered the most promising candidates. As a consequence, ten of these protein spots were selected (Fig. 3e, 2D gel protein spots U1-U10) for further experiments.
Dermanyssus gallinae 2D SDS PAGE and Western blots. Analysis of 2D SDS PAGE loaded with PRM total extracts stained with Coomassie blue (a and e) and Western blots (b–d). For Western blots IgY isolated from hens immunized with PRM total extracts and two different adjuvants (F Freund’s adjuvant and M Montanide ISA 70 VG) were used as primary antibody. Control IgY-C (b) shows IgY isolated from hens immunized without PRM extract. After analysis of the original Coomassie 2D SDS gels (a) and Western blot films (b–d), 2D gel protein spots recognized (e) by IgY preparations displaying anti-mite activity were excised and processed by proteomic analysis (U1-U10 mark the respective positions of these 2D gel protein spots)
Proteomics and vaccine candidate sequences
The selected 2D gel protein spots (Fig. 3e) were subjected to proteomic MS/MS. For the identification of PRM proteins, the MS/MS spectra were matched with PRM protein sequences, deduced from genomic and transcriptomic assemblies derived by high-throughput sequencing of total PRM DNA and RNA (data not shown). Eight PRM proteins were identified as unique protein sequences and potential vaccine candidates (Table 2), and their sequences were submitted to NCBI GenBank. Through matching of MS/MS spectra to proteins predicted from the sequenced transcriptome, the sequences from three PRM antigens were partially determined, while five of the eight PRM sequences were completely identified.
Sequence alignments were made through a search of these PRM sequences against all entries in the NCBI (GenBank) database. The vaccine candidate antigens were named PRM-1 to PRM-8 (Table 2) and have a high sequence homology with proteins from the predatory mite Metaseiulus occidentalis. Some of the proteins were only found in one 2D gel spot, whereas others were identified in several 2D gel proteins spots.
Discussion
In this study, we demonstrate that immunization of chicken with PRM extracts led to an increased mortality effect of the resulting IgY towards PRM in in vitro feeding assays. The identification of proteins which are contained in these extracts and which are recognized by such IgY preparations as protein spots in 2D-protein gels led to a number of potential candidates for vaccine development against PRM.
It has been shown previously that immunization of hens with PRM protein extracts leads to the generation of antibodies which cause mite mortality after in vitro feeding of PRM, and our results are in accordance with the effects observed in such studies (McDevitt et al. 2006; Harrington et al. 2009b; Wright et al. 2009). In addition, proteins present in extract fractions leading to an increased PRM mortality upon immunization were previously identified, but these attempts were complicated by the low resolution of standard one-dimensional protein gels, where usually several proteins are present in a single band and housekeeping proteins might be overrepresented (Harrington et al. 2009b).
An advantage of using 2D gels is the possibility to visualize individual protein spots. A potential pitfall of 2D gels is the fact that hydrophobic proteins, e.g., transmembrane or membrane-associated proteins, do not migrate well into both dimensions. We addressed this by using protocols for the analysis of GPI-anchored proteins (Fivaz et al. 2000); however, the resulting gels and Western blots were not significantly different (data not shown). After incubation of the blotted 2D gels with different antibody-preparations from immunized hens, the binding patterns of these antibodies were analyzed, and signals specific for IgY-fractions containing anti-PRM activity were identified.
We started the study by immunizing hens with PRM extracts formulated with two different adjuvants. It is known that different adjuvants stimulate preferentially the cellular or the humoral immune response, or both, hence, lead to different levels of antibody production (Stills 2005; Baz et al. 2012; Magnusson et al. 2014). For a vaccine against PRM, the humoral immune response is crucial, as the antibodies are taken up during the blood meal and need to find their targets in the parasite. Several previous studies have shown that the amounts of antibodies produced vary in direct comparisons between different Montanide adjuvants and Freund’s adjuvants (Bowden et al. 2003; Johansson and Hellman 2007; Roohvand et al. 2007; Babu et al. 2008; Marcq et al. 2015). In addition, Montanide ISA70 VG was shown to induce less unwanted side effects than Freund’s adjuvant when used in laying hens (Marcq et al. 2015). Hence, in order to identify the way to induce the maximum amount of antibodies displaying anti-PRM activity, both Freund’s and Montanide ISA70 VG were used in the present study.
PRM extracts formulated with both adjuvants led to an increase in PRM specific antibody responses, but the total amount of antibodies was higher for the Montanide ISA 70 VG adjuvant (Fig. 1). While a difference in antibody production between the adjuvants is in line with the studies cited above, the exact reasons for the better performance of Montanide ISA70 VG are beyond the scope of this investigation. Interestingly, not only the total amount of antibodies produced but also the pattern of antigens recognized (Fig. 3) differed between the two adjuvant groups. Again, the reason for this result remains to be determined; one potential explanation might include differing rates of antigen presentation via depot-effects of the adjuvants (Herbert 1967).
The difference in the outcome of both immunizations was used to identify protein spots associated with anti-PRM activity, namely, those that are exclusively or to a larger extent recognized by antibodies from hens immunized with the Montanide-adjuvanted extract. However, it cannot be excluded that proteins of relevance are also recognized by antibodies from the Freund’s-adjuvanted group; therefore, the initial selection of spots should be seen as the first attempt to identify antigens of interest.
Recently, Bartley et al. (2015) have published a similar study and identified PRM proteins which elicited antibodies with anti-PRM activity in in vitro feeding assays. Interestingly, only one protein (PRM-3 described here) was found in both studies. Possible explanations for the identification of different proteins might include differences in the antigen preparations used for initial immunizations. Bartley et al. (2015) used only the soluble protein fraction, whereas in the present study a mixture of soluble and insoluble proteins was applied. Therefore, the production of a different antibody population according to the kind of extract used for immunization is a possible explanation. This implies that the identification of potential vaccine antigens is highly dependent on the exact procedure used and underlines the need for in vivo challenge studies using chicken immunized with these proteins.
The results of the present study demonstrate that using a combination of immunization, in vitro-feeding experiments and proteomic approaches, a number of PRM-proteins were identified which are specifically recognized by antibody preparations causing mortality in poultry red mites. The group is very heterogeneous and includes unknown proteins as well as homologues to housekeeping proteins from other mite species. Based only on the sequences, it is not possible to judge which one of these proteins are suitable antigens for vaccination against PRM. However, the method presented here reduces the numbers of potential candidate antigens from several thousands (i.e., the PRM proteome) to a much smaller number which is feasible to be analyzed individually. Further immunization studies in chicken will provide more details for each one of the identified proteins.
References
Arkle S, Harrington D, Kaiser P, Rothwell L, De Luna C, George D, Guy J, Sparagano OAE (2008) Immunological control of the poultry red mite. Ann NY Acad Sci 1149:36–40
Babu JP, Pattnaik P, Gupta N, Shrivastava A, Khan M, Rao PL (2008) Immunogenicity of a recombinant envelope domain III protein of dengue virus type-4 with various adjuvants in mice. Vaccine 26:4655–4663
Bartley K, Wright HW, Huntley JF, Manson ED, Inglis NF, McLean K, Nath M, Bartley Y, Nisbet AJ (2015) Identification and evaluation of vaccine candidate antigens from the poultry red mite (Dermanyssus gallinae). Int J Parasitol 45:819–830
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Baz M, Samant M, Zekki H, Tribout-Jover P, Plante M, Lanteigne AM, Hamelin M-E, Mallett C, Papadopoulou B, Boivin G (2012) Effects of different adjuvants in the context of intramuscular and intranasal routes on humoral and cellular immune responses induced by detergent-split A/H3N2 influenza vaccines in mice. Clin Vaccine Immunol 19:209–218
Birkett MA, Hassanali A, Hoglund S, Pettersson J, Pickett JA (2011) Repellent activity of catmint, Nepeta cataria, and iridoid nepetalactone isomers against Afro-tropical mosquitoes, ixodid ticks and red poultry mites. Phytochemistry 72:109–114
Bowden TJ, Adamson K, MacLachlan P, Pert CC, Bricknell IR (2003) Long-term study of antibody response and injection-site effects of oil adjuvants in Atlantic halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol 14:363–369
Brockis DC (1980) Mite infestations. Vet Rec 107:315–316
Carroll JF (1994) Feeding deterrence of northern fowl mites (acari: Macronyssidae) by some naturally occurring plant substances. Pestic Sci 41:203–207
Chabierski S, Makert GR, Kerzhner A, Barzon L, Fiebig P, Liebert UG, Papa A, Richner JM, Niedrig M, Diamond MS, Palù G, Ulbert S (2013) Antibody responses in humans infected with newly emerging strains of West Nile Virus in Europe. PLoS One 8:e66507. doi:10.1371/journal.pone.0066507
Chauve C (1998) The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Vet Parasitol 79:239–245
Chirico J, Tauson R (2002) Traps containing acaricides for the control of Dermanyssus gallinae. Vet Parasitol 110:109–116
Craig R, Beavis RC (2004) TANDEM: matching proteins with tandem mass spectra. Bioinformatics 20:1466–1467
Fivaz M, Vilbois F, Pasquali C, van der Goot FG (2000) Analysis of glycosyl phosphatidylinositol-anchored proteins by two-dimensional gel electrophoresis. Electrophoresis 21:3351–3356
George DR, Masic D, Sparagano OAE, Guy JH (2009) Variation in chemical composition and acaricidal activity against Dermanyssus gallinae of four eucalyptus essential oils. Exp Appl Acarol 48:43–50
Gibb S (2014) MALDIquantForeign: Import/Export routines for MALDIquant. Chicago
Gibb S, Strimmer K (2012) MALDIquant: a versatile R package for the analysis of mass spectrometry data. Bioinformatics 28:2270–2271
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson D, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652
Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood P, Bowden J, Couger M, Eccles D, Li B, Lieber M, MacManes MD, Ott M, Orvis J, Pochet N, Strozzi F, Weeks N, Westerman R, William T, Dewey CN, Henschel R, LeDuc RG, Friedman N, Regev A (2013) De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512
Harrington D, Canales M, de la Fuente J, de Luna C, Robinson K, Guy J, Sparagano OAE (2009a) Immunisation with recombinant proteins subolesin and Bm86 for the control of Dermanyssus gallinae in poultry. Vaccine 27:4056–4063
Harrington D, El Din HM, Guy J, Robinson K, Sparagano OAE (2009b) Characterization of the immune response of domestic fowl following immunization with proteins extracted from Dermanyssus gallinae. Vet Parasitol 160:285–294
Herbert WJ (1967) Some investigations into the mode of action of the water-in-mineral-oil emulsion antigen adjuvants. In: Symposium Series of Immunobiology Standardization, Karger, Basel, NY:213–220
Herbert B, Galvani M, Hamdan M, Olivieri E, MacCarthy J, Pedersen S, Righetti PG (2001) Reduction and alkylation of proteins in preparation of two-dimensional map analysis: why, when, and how? Electrophoresis 22:2046–2057
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Johansson J, Hellman L (2007) Modifications increasing the efficacy of recombinant vaccines; marked increase in antibody titers with moderately repetitive variants of a therapeutic allergy vaccine. Vaccine 25:1676–1682
Jonsson NN, Matschoss AL, Pepper P, Green PE, Albrecht MS, Hungerford J, Ansell J (2000) Evaluation of TickGARDPLUS, a novel vaccine against Boophilus microplus, in lactating Holstein-Friesian cows. Vet Parasitol 88:275–285
Kamau LM, Wright HW, Nisbet AJ, Bowman AS (2013) Development of an RNA-interference procedure for gene knockdown in the poultry red mite, Dermanyssus gallinae: Studies on histamine releasing factor and Cathepsin-D. Afr J Biotechnol 12:1350–1356
Kilpinen O, Steenberg T (2009) Inert dusts and their effects on the poultry red mite (Dermanyssus gallinae). Exp Appl Acarol 48:51
Kirkwood AC (1967) Anaemia in poultry infested with the red mite Dermanyssus gallinae. Vet Rec 80:514–516
Lesna I, Sabelis MW, van Niekerk TGCM, Komdeur J (2012) Laboratory tests for controlling poultry red mites (Dermanyssus gallinae) with predatory mites in small “laying hen” cages. Exp Appl Acarol 58:371–383
Magnusson SE, Karlsson KH, Reimer JM, Corbach-Söhle S, Patel S, Richner JM, Nowotny N, Barzon L, Bengtsson KL, Ulbert S, Diamond MS, Stertman L (2014) Matrix-M™ adjuvanted envelope protein vaccine protects against lethal lineage 1 and 2 West Nile virus infection in mice. Vaccine 32:800–808. doi:10.1016/j.vaccine.2013.12.030
Marcq C, Marlier D, Beckers Y (2015) Improving adjuvant systems for polyclonal egg yolk antibody (IgY) production in laying hens in terms of productivity and animal welfare. Vet Immunol Immunopathol 165:54–63
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12
McDevitt R, Nisbet AJ, Huntley JF (2006) Ability of a proteinase inhibitor mixture to kill poultry red mite, Dermanyssus gallinae in an in vitro feeding system. Vet Parasitol 141:380–385
Moro CV, De Luna CJ, Alexander T, Guy JH, Sparagano OAE, Zenner L (2009) The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Exp Appl Acarol 48:93–104
Pohler P, Lehmann J, Veneruso V, Tomm JV, Bergen M, Lambrecht B, Kohn B, Weingart C, Seltsam A (2012) Evaluation of the tolerability and immunogenicity of UVC-irradiated autologous platelets in a dog model. Transfusion 52:2414–2426
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
R Core Team: (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Rice P, Longden I, Bleasby A (2000) EMBOSS: the European molecular biology open software suite. Trends Genet 16:276–277
Roohvand F, Aghasadeghi MR, Sadat SM, Budkowska A, Khabiri AR (2007) HCV core protein immunization with Montanide/CpG elicits strong Th1/Th2 and long-lived CTL responses. Biochem Biophys Res Commun 354:641–649
Sauer JR, McSwain JL, Essenberg RC (1994) Cell membrane receptors and regulation of cell function in ticks and blood-sucking insects. Int J Parasitol 24:33–52
Schicht S, Qi W, Poveda L, Strube C (2013) The predicted secretome and transmembranome of the poultry red mite Dermanyssus gallinae. Parasit Vectors 6:259
Schicht S, Qi W, Poveda L, Strube C (2014) Whole transcriptome analysis of the poultry red mite Dermanyssus gallinae (De Geer, 1778). Parasitology 141:336–346
Sechi S, Chait BT (1998) Modification of cysteine residues by alkylation. A tool in peptide mapping and protein identification. Anal Chem 70:5150–5158
Sparagano OAE (2009) Control of poultry mites: where do we stand? Exp Appl Acarol 48:1–2
Sparagano OAE, Pavlićević A, Murano T, Camarda A, Sahibi H, Kilpinen O, Mul M, van Emous R, le Bouquin S, Hoel K, Cafiero MA (2009) Prevalence and key figures for the poultry red mite Dermanyssus gallinae infections in poultry farm systems. Exp Appl Acarol 48:3–10
Steenberg T, Kilpinen O (2014) Synergistic interaction between the fungus Beauveria bassiana and desiccant dusts applied against poultry red mites (Dermanyssus gallinae). Exp Appl Acarol 62:511–524
Stills HF (2005) Adjuvants and antibody production: dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J 46:280–293
van Emous R (2005) Wage war against the red mite! Poultry Int 44:26–33
Wickham H (2011) The split-apply-combine strategy for data analysis. J Stat Softw 40:1–29
Willadsen P, Kemp DH (1988) Vaccination with ‘concealed’ antigens for tick control. Parasitol Today 4:196–198
Willadsen P, Riding GA, McKenna RV, Kemp DH, Tellam RL, Nielsen JN, Lahnstein J, Cobon GS, Gough JM (1989) Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J Immunol 143:1346–1351
Wright HW, Bartley K, Nisbet AJ, McDevitt RM, Sparks NH, Brocklehurst S, Huntley JF (2009) The testing of antibodies raised against poultry red mite antigens in an in vitro feeding assay; preliminary screen for vaccine candidates. Exp Appl Acarol 48:81–91
Acknowledgments
We thank Steffen Jakob, Ulrike Ehlert, and Maria Aulmann for the excellent technical assistance. We also thank Drs. Jasmin Fertey and Beyene Moges Agizie for critical reading of the manuscript and support in the statistical analysis. We thank Dr. Daniela Volke (University of Leipzig) for the analysis of PRM 2D gel protein spots.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors’ contributions
GRM and SU conceived, designed and coordinated the study. GRM performed the experiments, SV carried out the immunizations. MEKJ and MV contributed expertise in birds and poultry experiment design. KS carried out essential sequencing of PRM DNA and RNA. TB performed bioinformatics and statistical analyses. GRM and SU analyzed and interpreted the data and drafted the manuscript. All authors have read and approved the final manuscript.
Conflict of interest
This study was funded by Lohmann Tierzucht GmbH. The funding body had no role in design, collection, analysis, and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Rights and permissions
About this article
Cite this article
Makert, G.R., Vorbrüggen, S., Krautwald-Junghanns, ME. et al. A method to identify protein antigens of Dermanyssus gallinae for the protection of birds from poultry mites. Parasitol Res 115, 2705–2713 (2016). https://doi.org/10.1007/s00436-016-5017-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5017-2