Abstract
Tegument antigens of tapeworm play an important role in modulation of host response and parasite survival. Characterizing appropriate antigens for parasite infection diagnosis and vaccination is rational and could have both economic and epidemiological significance in poultry industry. In the present study, a major protoscolex homologue (named RT10) of Echinococcus and Taenia spp. was amplified from Raillietina tetragona cestode. The RT10 cDNA was 1,877 bp long containing an open reading frame of 1,683 bp nucleotides, which encoded a deduced protein of 560 amino acids with an isoelectric point of 6.33. Secondary structure analysis demonstrated that RT10 was both hydrophilic and antigenic, and possessed N-terminal FERM domain and C-terminal ERM domain, respectively. With the same structural properties of previously reported antigens from Echinococcus and Taenia spp., RT10 tegument antigen had a more than 82 % similarity in nucleotide level with initially reported antigens from Echinococcus and Taenia spp., and a more than 83 % similarity in protein level, with the highest similarity of 85.2 % to Taenia antigen H17g. In addition, phylogenetic analysis illustrated a high consistency between different genus antigens and evolutionary branching. Although the detailed function of RT10 is still unknown, the high sequence conservation and structural similarity to formerly identified tegument antigens from Echinococcus and Taenia spp. suggested that RT10 may play a similar role as the previous reported antigens between cestode and host. It is significant to clarify the antigenic and serodiagnostic characteristics in the subsequent work.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cestode tapeworm infection brought great economic losses to the poultry industry, especially in developing areas, such as Africa and South Asia (Abdelqader et al. 2008; Hassouni and Belghyti 2006; Puttalakshmamma et al. 2008; Phiri AMP et al. 2007; Magwisha et al. 2002; Permin et al. 1997; Poulsen et al. 2000; Shah et al. 1999; Salam et al. 2010). These losses were in forms of reduced weight gain, retarded growth, decreased egg production, obstruction of intestine, diarrhea, morbidity and mortality (Anwar et al. 1991). The cestode infection rates were high and varied a lot depending on geographical areas and climate conditions. Tapeworm infections reported from traditional poultry were mainly the genus of Raillietina cestodes (Salam et al. 2010). The intermediate hosts of the parasite are mainly the ants which carry infectious cysticercoids of the Raillietina cestodes. The chickens become infected with Raillietina tetragona as a result of eating ants which contain the infectious cysticercoids of the cestode (Horsfall 1938). Anti-tapeworm drugs such as niclosamide and pyquiton may be effective to eradicate the parasite infections in epidemiological areas. However, free-ranging keeping of chicken and poor management, together with less use of these anti-tapeworm drugs, were reasons for the high infection rates in these developing areas (Avsatthi and Varghese 1981; Hemalatha et al. 1987). Therefore, finding possible antigens for diagnosis and prevention against tapeworm infections through vaccination could have both epidemiological and economic significance in these developing areas.
The parasites’ surface antigens are logical candidates for vaccine development and immunodiagnostic utilities. The major protoscolex surface antigens of Echinococcus multilocularis (EM10) and Echnococcus granulosus (EG10) were originally characterized (Frosch et al. 1991; Frosch et al. 1994). Several groups had also identified truncated cDNA clones similar to EM10 and EG10, namely, EMII/3 and EGII/3 (Felleisen and Gottstein 1994), EM4 (Hemmings and McManus 1991), and EM18 (Sako et al. 2002). Taenia homologues of the major surface antigen of Echinococcus spp. R-Tso18 (also named TEG-Tsag, Taenia saginata) and H17g (also named TEG-Tsol, Taenia solium) were characterized (Benitez et al. 1998; Ferrer et al. 2003; Gonzalez et al. 2007). The full length and the truncated major surface antigens reported by the previous groups showed the antigens possessed immunogenic properties and had serodiagnostic significance. However, the similar major protoscolex surface antigen in Raillietina genus had never been reported so far as we knew. To address the question, we had cloned a 1,877-bp cDNA sequence encoding a putative protein of 560 amino acids from cestode R. tetragona. Further multi-sequence alignment revealed that RT10 had more than 82 % similarity to those of Echinococcus and Taenia spp. antigens both in nucleotide and protein levels. The high sequential and structural similarities demonstrated that RT10 may be immunogenic as well, and future work will be focused on production of anti-RT10 antibody and investigation of the immune response of the RT10 between the parasite and the host.
Materials and methods
Sampling and extraction of total RNA from tapeworm
Adult R. tetragona tapeworms were obtained by dissecting chicken intestines purchased from Sanjiao market of South China Agricultural University, Guangdong, China. After washing three times with phosphate buffer saline (PBS), the tapeworms were microscopically photographed and preserved in liquid nitrogen in aliquots for RNA extraction. The tapeworm was crushed with pestle and lysed using RNAisoTMPlus total RNA extraction reagent (Takara, Dalian, China). The procedure of total RNA extraction was performed according to the manufacturer’s standard instruction.
Reverse transcription of total mRNA
After being quantified and qualified respectively by spectrophotography and agarose gel electrophoresis stained with ethidium bromide, the total RNA from R. tetragona was reverse-transcribed using M-MLV reverse transcriptase (Promega) according to the manufacturer’s instruction.
Amplification of partial sequence of RT10 antigen cDNA
Six tegumental antigen mRNA sequences, namely, EM10, EG10, EMII/3, EGII/3, H17g and R-Tso18 from four species, were multi-aligned using Clustal X software (Thompson et al. 1997) to find out a homologous sequence for designing a pair of polymerase chain reaction (PCR) primers using primer premier 5.0 software. The primers used to amplifying the partial sequence of RT10 antigen cDNA were as follows: forward primer, 5′ GGGTTTGAGTATTTACGAGCC 3′; reverse primer, 5′ GGTGCAAAGAGCCAAGATG 3′. PCR was performed using the following program: initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 30 s, annealing at 46 °C for 30 s, extension at 72 °C for 30 s, and a final extension at 72 °C for 10 min. The DNA product was examined by agarose gel electrophoresis and ethidium bromide staining. Subsequently, the partial DNA product was purified using an AXYGEN kit and cloned into pGM-T plasmid (Tiangen, China). The recombinant plasmid then was transformed into DH5α competent bacteria, and the partial DNA in the plasmid was sequenced by Invitrogen using the general primers (T7/T7T).
3′ Terminal sequence amplification of RT10 cDNA
The 3′ terminal sequence of RT10 antigen was amplified by use of RACE (rapid amplification of cDNA ends (Frohman et al. 1988) method. As previously described, the reverse transcription except of total RNA from R. tetragona was performed using another primer 5′ GGCCACGCGTCGACTAGT ACTTTTTTTTTTTTTTTTTTT 3′. The reversely transcribed product was diluted for PCR amplification, and a pair of specific PCR primer was generated using Primer Premier 5.0 software according to the previous partial RT10 cDNA sequence. The specific primers were as follows: forward primer, 5′ CCTGGGTTTGAGTATTTACGAGC 3′; reverse primer, 5′ GGCCACGCGTCGACTAGTAC 3′. The PCR reaction program was as follows: 5 min for initial denaturation, 30 cycles of denaturation at 94 °C for 30 s, annealing at 65 °C for 30 s, extension at 72 °C for 90 s, and 10 min for the last extension. The PCR product was subjected to electrophoretic purification and ligated into pGM-T plasmid for sequencing.
5′ Partial sequence amplification
Six tegument antigen sequences from species mentioned above were multi-aligned, and a conserved sequence located at start codon was used to design a forward degenerate primer as 5′ ATGTTGAA GAGGRGTAAGAAT 3′, where “R” stands for A or G nucleotide. However, the backward primer used to amplify the 5′ partial sequence was the same backward primer used in cloning the previously partial sequence. The sequence of the PCR program was initial denaturation at 94 °C for 5 min followed by 30 cycles at 94 °C for 30 s, annealing at 62 °C for 30 s, extension at 72 °C for 60 s, and lastly, 10 min for extension. Subsequently, purification and sub-cloning, together with sequencing of PCR product of 5′ partial sequence, were performed as previously described.
Sequences assembly and bioinformatic analysis of the RT10 antigen
The DNA sequence assembly of RT10 with the three partial sequences amplified previously was carried out using Seqman of Lasergene software packages (DNASTAR, Madison, WI, USA). Thereafter, cDNA sequences including open reading frame, 3′ untranslated region, as well as polyadenylation signal were analyzed. Assembled RT10 cDNA sequence was deposited in the NCBI GenBank. With the predicted amino acid sequences encoded by RT10 antigen, the molecular mass, isoelectric point, along with amino acid composition were studied by use of Protean of Lasergene software packages (DNASTAR, Madison, WI, USA). Furthermore, protein sequence was entered into websites http://www.cbs.dtu.dk/services/TMHMM (Krogh et al. 2001) for transmembrane analysis and http://www.cbs.dtu.dk/services/SignalP/ (Bendtsen et al. 2004) for putative signal peptide expression analysis. The RT10 antigen was analyzed for potential domains by NCBI Conserved Domain Search program. Protein secondary structure and antigenic property, along with hydrophilic characteristic, were analyzed using Protean of Lesergene software packages (DNASTAR, Madison, WI, USA). Nucleotide and amino acid sequences of RT10 tegument antigen together with the antigens of the corresponding species mentioned above were further multi-aligned using Clustal X software to see their similarities. Furthermore, the protein sequences aligning result was subsequently processed to construct a phylogenetic tree using Mega 3.0 software (Kumar et al. 2004) by neighbor-joining model, and bootstrap analysis was done for the tree.
Identification of full-length cDNA encoding RT10 protein in R. tetragona
Based on the RT10 full-length sequence assembled previously, a pair of PCR primer was generated to amplify the full coding sequence of RT10 antigen. The PCR product was further sub-cloned into pGM-T vector (Tiangen, China) for sequencing. The primers used to amplify the full coding sequence were as follows: forward primer, 5′ ATGTTGAAGAGGGGTAAG 3′; backward primer, 5′ CATGGACTCAAACTGCTC 3′.
Results
Amplification of the partial sequence of RT10 antigen
The R. tetragona tapeworms were collected from chicken intestines, and an approximately 200 bp nucleotides fragment (Fig. 1, lanes 1 to 5) of RT10 cDNA was amplified using a pair of PCR primers designed from the multiple-aligned results (not shown) between six tapeworm tegument antigen sequences from GenBank, namely, EM10 (E. multilocularis, accession number M61186), EMII/3 (E. multilocularis, accession number U05573), EG10 (Echnococcus granulosus, accession number: Z29489), EGII/3 (E. granulosus, accession number: U05574), H17g (T. solium, accession number AJ581299), R-Tso18 (T. saginatus, accession number X97000). Subsequently, sequencing of the partial RT10 sequence by sub-cloning into pGM-T plasmid and the sequencing results for blasting on NCBI nr database certified our expectation that the amplified sequence was the targeted partial fragment of the RT10 sequence (data not shown).
PCR amplification of tegument antigen RT10 from R. tetragona cestode. Lanes 1 to 5 were partial cDNA sequence of RT10 antigen in R. tetragona; lane 6 was the 3′ terminal sequence of RT10 antigen amplified by means of 3′ RACE methods; lane 7 showed the 5′ partial sequence terminated at start codon of RT10; lanes 8 to 11 represented the full-length RT10 coding sequence PCR product; molecular mass of different bands was as indicated respectively
3′ RACE and 5′ partial sequences amplification
3′ RACE method was performed to amplify the 3′ terminal sequence of RT10 antigen in R. tetragona (Fig. 1, lane 6). Sequencing result showed the 3′ terminated sequence was a 1,200 bp nucleotide fragment (data not shown). Specifically, in the amplification of 5′ partial sequence (Fig. 1, lane 7), a specific primer and a degenerate primer were involved, and sequencing result showed a 860 bp fragment of 5′ partial sequence (data not shown). Multi-alignment of 3′ RACE or 5′ partial sequence with previously reported corresponding antigens from Echinococcus and Taenia spp. demonstrated a high consistency with these homologues from NCBI GenBank (data not shown).
Bioinformatic analysis of RT10 antigen sequence
The 3′ terminal sequence, 5′ partial sequence, and firstly amplified partial sequence were assembled to obtain a total of 1,877 bp nucleotide sequence of RT10 antigen using Seqman of Lasergene package software. With the assembled sequence, the whole coding sequence was amplified to verify the existence of RT10 antigen (Fig. 1, lanes 8–11). Analysis of RT10 antigen nucleotide sequence and comparison with those antigens from other species mentioned above in GenBank revealed that RT10 antigen had a single open reading frame of 1,683 bp, followed by a 3′ untranslated region of 194 bp, which included a possible eukaryotic consensus polyadenylation signal AATAAA, 16-nucleotide upstream from the poly(A)+ tail. However, RT10 cDNA sequence was incomplete as we did not clone the terminus of the 5′ untranslated region. Multiple alignment of other antigens indicated the 5′ untranslated region range from 25 to 45 bp. Subsequently, the nucleotide sequence of RT10 was deposited into NCBI GenBank and is available with accession number HQ858609. The potential open reading frame of RT10 encoded a full-length protein of 560 amino acid residues, with a theoretical molecular mass of approximately 65.4 kDa and an isoelectric point of 6.53. Online Conserved Domain Searching (NCBI) analysis (Fig. 2, top) showed that RT10 antigen involved three superfamilies, which from N-terminal to C-terminal were literally erzin-radixin-moesin (FERM)_N superfamily, FERM_M superfamily, FERM_C superfamily, and two multi-domains: B41 and ERM, respectively. Putative RT10 polypeptide analysis showed that RT10 protein consisted of an extensive α-helical regions and a long hydrophilic sequence (Fig. 2, down). Analysis using SignalP 3.0 server prediction showed that the RT10 did not contain signal peptide (data not shown). The prediction of transmembrane regions indicated that none of transmembrane region existed in RT10 antigen polypeptide (data not shown).
Locations of FERM superfamilies, multi-domains (B41 and ERM), secondary structure (involving α-helix, β-sheet, turn, and coil), hydrophilicity plot, and antigenic index, and surface probability plot in putative RT10 protein. Numbers indicate the amino acid positions. RT10 structure was analyzed by Conserved Domain Search (NCBI) and Lesergene software packages (DNASTAR)
Sequence similarity and phylogenetic analysis of RT10 antigen
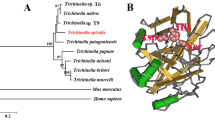
Multiple alignment of RT10 nucleotide (data not shown) and deduced amino acid sequences with its corresponding homologues from the other species were performed (Fig. 3). The percentage similarities between RT10 and other homologues were shown in Table 1, and the results demonstrated a highest similarity (85.2 %) between RT10 and H17g in protein level. In addition, the percentage similarities were all higher than 83 % with the other six antigens in the protein level and more than 82 % in the nucleotide level. However, a non-tegument antigen JF-2 (Kurtis et al. 1997) from Schistosoma japonicum was much less pronounced (Table 1). Furthermore, protein alignment result was used to construct a phylogenetic tree using neighbor-joining model, and the bootstrap test also was performed (Fig. 4). On the tree, the RT10 protein sequence was divided into single branch; the Taenia species molecules (including H17g and R-Tso18 molecules) were located into a branch; while the Echinococcus species molecules (EM10, EMII/3, EG10, EGII/3) were affiliated to the third branch.
Multiple alignment of the deduced amino acid sequences: RT10 of R. tetragona (HQ858609), H17g (TEG-Tsol) of T. solium (AJ581299), R-Tso18(TEG-Tsag) of T. saginata (X97000), EM10 of E. multilocularis (M61186), EMII/3 of E. multilocularis (U05573), EG10 of E. granulosus (Z29489), EGII/3 of E. granulosus (U05574). The alignment was generated using the Clustal X software. The dots represent the same amino acid
Discussion
Tapeworm infection brought great economic losses to poultry industry, especially in a backyard free ranging environment where chicken were more susceptible to parasite infections via litter droppings and scavenging habits (Puttalakshmamma et al. 2008). Finding new tegument antigens for parasite diagnosis or vaccination may be both economic and epidemiologically significant in the poultry industry. In the present study, we characterized a similar protoscolex tegument antigen RT10 from R. tetragona through analysis of the similarities in both amino acid and nucleotide sequences of protoscolex tegument antigens from Echinococcus and Taenia cestodes. The bioinformatic analysis showed more than 82 % similarities between RT10 and Echinococcus or Taenia spp. antigens in both nucleotide and amino acid level. The secondary structure prediction showed that RT10 possessed N-terminal FERM domain and C-terminal ERM domain, which is the same structure with previous reports (Gonzalez et al. 2007), suggesting that RT10 could function as a link between membrane and cytoskeleton as well in R. tetragona cestode (Lankes and Furthmayr 1991). Secondary structure prediction of RT10 demonstrated it as a hydrophilic molecule, which is the same with the previously reported antigens from Echinococcus and Taenia spp. As a whole, we can conclude that this putative RT10 antigen could be a hydrophilic and soluble molecule possessing crucial importance for modulation of host response and parasite survival (Gonzalez et al. 2007).
The phylogenetic analysis of RT10 protein sequence with previously reported antigens from Echinococcus and Taenia spp. suggested a high evolutionary consistency between the three genera, namely, each genus was divided into a single branch. However, the protein sequence alignment of RT10 revealed great differences between 340 and 460 amino acids with the other four species (Fig. 3). Secondary structure demonstrated that this region is the major sequence of ERM domain (Fig. 2). Previous reports implicated that the functions of ERM family were cell-to-cell adherence junctions, cleavage furrows, microvilli, and ruing membranes (Sato et al. 1992). However, whether the ERM domain differences between RT10 and the other six tegument antigens possess distinctive functions remained to be further investigated.
Although the detailed function of the RT10 antigen is unknown, the striking homology with antigens from Echinococcus and Taenia spp. raises the possibility that it could be a tegument/surface protein, perhaps associated with parasite growth and mutual interaction between parasite and host (Felleisen and Gottstein 1994; Frosch et al. 1991). In addition, protoscolex antigens and truncated peptides previously reported in Echinococcus and Taenia spp. were regarded as an excellent diagnostic reagent for both human and animal oncosphere infections (Ferrer et al. 2003; Gonzalez et al. 2007; Helbig et al. 1993; Kouguchi et al. 2005; Sako et al. 2002; Vogel et al. 1988). It is significant to study the potential utilities of RT10 as a serodiagnostic antigen in chicken tapeworm infections. The future work will be focused on the production of anti-RT10 antibody, serodiagnostic examination of the tapeworm infection in chickens, and crucially the antigen localization of the parasite.
References
Abdelqader A, Gauly M, Wollny CBA, Abo-Shehada MN (2008) Prevalence and burden of gastrointestinal helminthes among local chickens, in northern Jordan. Pre Vet Med 85(1–2):17–22
Anwar AH, Hayat S, Hayat CS (1991) Prevalence of gastrointestinal parasitic fauna of indigenous and exotic layer chickens in and around Faisalabad. Pakistan Vet J 1:9–12
Avsatthi BL, Varghese K (1981) Efficacy of compound SRC-4402(2-chloro-4 isthiocyananto bresorcylanilid) against poultry cestodes. Indian J Poul Sci 16(1):286–287
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340(4):783–795
Benitez L, Harrison LJS, Parkhouse RME, Gonzalez LM, Gottstein B, Garate T (1998) Sequence and immunogenicity of the Taenia saginata homologue of the major surface antigen of Echinococcus spp. Parasitol Res 84(5):426–431
Felleisen R, Gottstein B (1994) Comparative analysis of full-length antigen II/3 from Echinococcus multilocularis and E. granulosus. Parasitol 109(2):223–232
Ferrer E, Benitez L, Foster-Cuevas M et al (2003) Taenia saginata derived synthetic peptides with potential for the diagnosis of bovine cysticercosis. Vet Parasitol 111(1):83–94
Frohman MA, Dush MK, Martin GR (1988) Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A 85(23):8998–9002
Frosch PM, Frosch M, Pfister T et al (1991) Cloning and characterisation of an immunodominant major surface antigen of Echinococcus multilocularis. Mol Biochem Parasitol 48(2):121–130
Frosch PM, Muhlschlegel F, Sygulla L et al (1994) Identification of a cDNA clone from the larval stage of Echinococcus granulosus with homologies to the E. multilocularis antigen EM10-expressing cDNA clone. Parasitol Res 80(8):703–705
Gonzalez LM, Ferrer E, Spickett A et al (2007) The Taenia saginata homologue of the major surface antigen of Echinococcus spp. is immunogenic and 97 % identical to its Taenia solium homologue. Parasitol Res 101:1541–1549
Hassouni T, Belghyti D (2006) Distribution of gastrointestinal helminths in chicken farms in the Gharb region—Morocco. Parasitol Res 99:181–183
Helbig M, Frosch P, Kern P et al (1993) Serological differentiation between cystic and alveolar echinococcosis by use of recombinant larval antigens. J Clin Microbiol 31(12):3211–3215
Hemalatha EA, Rehman SA, Jagannath MS (1987) Helminth infection in domestic fowls reared on deep litter and cage system. Mysore J Agri Sci 21:338–341
Hemmings L, McManus DP (1991) The diagnostic value and molecular characterisation of an Echinococcus multilocularis antigen gene clone. Mol Biochem Parasitol 44(1):53–61
Horsfall MW (1938) Observations on the life history of Raillietina echinobothrida and of R. tetragona (Cestoda). J Parasitol 24(5):409–421
Kouguchi H, Suzuki T, Yamano K et al (2005) Characterization of various recombinant antigens from Echinococcus multilocularis for use in the immunodiagnosis. Protein Journal 24(1):57–64
Krogh A, Larsson B, von Heijne G et al (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305(3):567–580
Kumar S, Tamura K, Nei M (2004) MEGA3: Integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief Bioinform 5(2):150–163
Kurtis JD, Ramirez BL, Wiest PM et al (1997) Identification and molecular cloning of a 67-kilodalton protein in Schistosoma japonicum homologous to a family of actin-binding proteins. Infect Immun 65(1):344–347
Lankes WT, Furthmayr H (1991) Moesin: a member of the protein 4.1-talin-ezrin family of proteins. Proc Natl Acad Sci U S A 88(19):8297–8301
Magwisha HB, Kassuku AA, Kyvsgaard NC et al (2002) A Comparison of the prevalence and burdens of helminth infections in growers and adult free-range chickens. Trop Anim Health and Pro 34(3):205–214
Permin A, Magwisha H, Kassuku AA et al (1997) A cross-sectional study of helminths in rural scavenging poultry in Tanzania in relation to season and climate. J Helminthol 71(03):233–240
Phiri AMP IK, Ziela M, Chota A et al (2007) Prevalence and distribution of gastrointestinal helminths and their effects on weight gain in free-range chickens in Central Zambia. Trop Anim Health Prod 39:309–315
Poulsen J, Permin A, Hindsbo O et al (2000) Prevalence and distribution of gastro-intestinal helminths and haemoparasites in young scavenging chickens in upper eastern region of Ghana, West Africa. Prev Vet Med 45(3–4):237–245
Puttalakshmamma GC, Ananda KJ, Prathiush PR, Mamatha GS, Rao S (2008) Prevalence of gastrointestinal parasites of poultry in and around Banglore. Vet World 1(7):201–202
Sako Y, Nakao M, Nakaya K et al (2002) Alveolar echinococcosis: characterization of diagnostic antigen Em18 and serological evaluation of recombinant Em18. J Clin Microbiol 40(8):2760–2765
Salam ST, Mir MS, Khan AR (2010) The prevalence and pathology of Raillietina cesticillus in indigenous chicken (Gallus gallus domesticus) in the temperate Himalayan region of Kashmir - short communication. Vet Arh 80(2):323–328
Sato N, Funayama N, Nagafuchi A et al (1992) A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci 103(Pt 1):131–143
Shah AH, Anwar AUH, Khan MNK et al (1999) Comparative studies on the prevalence of cestode parasites in indigenous and exotic layers at Faisalabad. Int J Agri Biol 1(4):277–279
Thompson JD, Gibson TJ, Plewniak F et al (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Vogel M, Gottstein B, Muller N et al (1988) Production of a recombinant antigen of Echinococcus multilocularis with high immunodiagnostic sensitivity and specificity. Mol Bio Parasitol 31(2):117–125
Acknowledgments
This work was supported by Guangdong Province Natural Science Fund (no. 06025794)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Li, H. Biochemical and molecular characterization of the tegument protein RT10 from Raillietina tetragona . Parasitol Res 113, 1239–1245 (2014). https://doi.org/10.1007/s00436-014-3763-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3763-6