Abstract
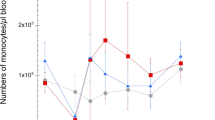
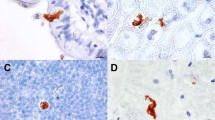
Chicken (Gallus gallus) were used as the experimental model for study of immune response against the microsporidium Encephalitozoon hellem (Didier et al., J Inf Dis 163:617–621, 1991) infection in birds. Two-day-old chicken were infected perorally or intraperitoneally with a dose of 107 spores of E. hellem. The anti-E. hellem immunoglobulin (Ig)A, IgY, and IgM antibody responses in sera and dropping sample extracts were determined by enzyme-linked immunosorbent assay. Results have shown specific antibody production in sera and intestinal secretions of infected birds. Chicken inoculated perorally developed the lowest antibody response. Microsporidian spores were not identified in the smears from cloacal swab samples of individual chicken. Intestinal segment cultures of perorally infected chicken cultivated in vitro showed the highest production of specific IgY and IgA antibodies in jejunum segments. In the further course of infection, the colon produced the highest amount of IgA, and the ileum and colon produced the highest amount of IgY.


Similar content being viewed by others
References
Barton CE, Phalen DN, Snowden KF (2003) Prevalence of microsporidian spores shed by asymptomatic lovebirds: evidence for potential emerging zoonosis. J Avian Med Surg 17:197–202
Black SS, Steinohrt LA, Bertucci DC, Rogers LB, Didier ES (1997) Encephalitozoon hellem in budgerigars (Melopsittacus undulatus). Vet Pathol 34:189–198
Bryan RT, Schwartz DA (1999) Epidemiology of microsporidiosis. In: Wittner M (ed) Microsporidia and microsporidiosis. ASM, Washington, DC, pp 502–530
Didier ES (1995) Reactive nitrogen intermediates implicated in the inhibition of Encephalitozoon cuniculi (phylum Microspora) replication in murine peritoneal macrophages. Parasite Immunol 17:405–412
Didier ES, Didier PJ, Friedberg DN, Stenson SM, Orenstein JM, Yee RW, Tio FO, Davis RM, Vossbrinck C, Millichamp N et al (1991) Isolation and characterisation of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitis. J Infect Dis 163:617–621
Didier ES, Varner PW, Didier PJ, Aldras AM, Millichamp NJ, Murphey-Corb M, Bohm R, Shadduck JA (1994) Experimental microsporidiosis in immunocompetent and immunodeficient mice and monkey. Folia Parasitol 41:1–11
Didier ES, Rogers LB, Brush AD, Wong S, Traina-Dosge V, Bertuccci D (1996) Diagnosis of disseminated microsporidian Encephalitozoon hellem infection by PCR–Southern analysis and successful treatment with albendazol and fumagillin. J Clin Microbiol 34:947–952
Diesenhouse M, Wilson L, Corrent G, Visvesvara G, Grossniklaus H, Bryan R (1993) Treatment of microsporidial keratoconjunctivitis with topical fumagillin. Am J Ophthalmol 15:293–298
Fayer R, Santin M, Palmer R (2003) Comparison of microscopy and PCR for detection of three species of Encephalitozoon in feces. J Eukaryot Microbiol 50:572–573
Gannon J (1980) The course of infection of Encephalitozoon cuniculi in immunodeficient and immunocompetent mice. Lab Anim 14:189–192
Gray ML, Puette M, Latimer KS (1998) Microsporidiosis in a young ostrich (Struthio camelus). Avian Dis 42:832–836
Hollister WS, Canning EU (1987) An enzyme-linked immunosorbent assay (ELISA) for detection of antibodies to Encephalitozoon cuniculi and its use in determination of infections in man. Parasitology 94:209–219
Kotler DP, Orenstein JM (1998) Clinical syndromes associated with microsporidiosis. Adv Parasitol 40:321–349
Loa CC, Lin TL, Wu CC, Bryan T, Hooper T, Schrader D (2002) Specific mucosal IgA immunity in turkey poults infected with turkey coronavirus. Vet Immun Immunopathol 88:57–64
Niederkorn JY, Shadduck JA (1980) Role of antibody and complement in the control of Encephalitozoon cuniculi infection in rabbit macrophages. Infect Immun 17:995–1002
Ochsenbein AF, Zinkernagel RM (2000) Natural antibodies and complement link innate and acquired immunity. Immunol Today 21:624–630
Pulparampil N, Graham D, Phalen D, Snowden K (1998) Encephalitozoon hellem in two eclectus parrots (Eclectus roratus): identification from archival tissues. J Eukaryot Microbiol 45:651–655
Rosberger D, Serdarevic G, Erlandson R, Bryan R, Schwartz D, Visvesvara G, Keenan PC (1993) Successful treatment of microsporidial keratoconjunctivitis with topical fumagillin in a patient with AIDS. Cornea 12:261–265
Schmidt EC, Shadduck JA (1984) Mechanisms of resistance to the intracellular protozoan Encephalitozoon cuniculi in mice. J Immunol 133:2712–2719
Silverstein B, Cunningham EJ, Margolis T, Cavallos V, Wong I (1997) Microsporidial keratoconjunctivitis in a patient without human immunodeficiency virus infection. Am J Ophthal Mol 124:395–396
Snowden K, Logan K (1999) Molecular identification of Encephelitozoon hellem in an ostrich. Avian Dis 43:779–782
Snowden K, Phalen DN (2004) Encephalitozoon infection in birds. Semin Avian Exot Pet Med 13:94–99
Acknowledgements
This study was supported by Ph.D. grant 524/03/H133 and a research project (Z60220518) of the Institute of Parasitology, Academy of Sciences of the Czech Republic. All experiments were done in accordance with the national law on the use of experimental animals, safety, and use of pathogenic agents.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saková, K., Sak, B., Ditrich, O. et al. Humoral response of chicken infected with the microsporidium Encephalitozoon hellem . Parasitol Res 98, 488–492 (2006). https://doi.org/10.1007/s00436-005-0089-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-0089-4