Abstract
Within meiofauna, cnidarians are represented by only a few species most of which are in the genus Halammohydra Remane, 1927. It represents highly modified medusae. Information about this group is limited, which complicates its placement in the Cnidarian tree and the relationship to another meiofaunal cnidarian, Otohydra Swedmark & Teissier, 1958. This needs to be clarified with molecular, but also with morphological methods. In this study, the internal organization of H. vermiformis Swedmark & Teissier, 1957 from Sylt and Helgoland (Germany) was examined using transmission electron microscopy (TEM). The ultrastructure of both sexes is documented in this study, i.e. the gastric tube including gonadal compartment, aboral cone, statocysts and tentacles. It is proposed that spermatozoa and oocytes are not released into the water through the gastrodermis, but by rupture of the epidermis, because of structural changes in the epidermis. In both, male and female, there is an indent in the gastric tube and a gap of the mesoglea at the same position. Additionally, we describe the complex structure of the aboral cone with the specialized adhesive organ as well as the accumulation of myofibrils and neurites in the orally directed part of the cone, which indicates high controllability and ability to move in this region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the diverse meiofaunal animals in marine sediments, there are few representatives of the phylum Cnidaria and they are dominated by Hydrozoans (Schmidt-Rhaesa et al. 2020). There is only one Staurozoan genus (Stylocoronella) and no representatives of Anthozoa and Cubozoa are known so far (Kikinger and Salvini-Plawen 1995). Within Hydrozoa, meiofaunal cnidarians can represent either the polyp or the medusa stage. Seven genera are attributable to the polyp stage and they are classified among the Hydroidolina. The first described meiofaunal cnidarian was Protohydra leuckarti Greeff, 1870, a polyp without tentacles and with the ability to drastically change body shape (Greeff 1870). The reduction of tentacles and the flexibility of the body are perfect adaptations to the interstitial system. With intensified investigations of sediment in the following years, more cnidarian representatives were discovered. Interestingly, not only polyps found their way into the sediment, but medusae, too. Presumably, medusa-derived taxa are found in four genera, all belonging to the subclass Trachylinae. The best-investigated group and the genus with the most known species (nine) is Halammohydra Remane, 1927. It was first discovered by Remane in 1927 in the Baltic Sea and on Helgoland in Germany, and then found in different locations in Europe, mainly Germany (e.g. Clausen 1967; Polte and Schmidt-Rhaesa 2011; Schmidt 1969; Swedmark and Teissier 1957), France (e.g. Swedmark and Teissier 1957, 1967; Teissier 1950), Norway (e.g. Clausen 1963, 2000) and United Kingdom (e.g. Boaden 1961, 1963). Other European locations are Sweden (Boaden 1960; Dahl 1953), Ireland (Boaden 1966), the Netherlands (Wolff et al. 1974), Italy (Salvini-Plawen 1991), Spain (Martínez et al. 2009; 2019) and Portugal (Tödter and Schmidt-Rhaesa 2021). Outside of Europe, Halammohydra was reported particularly from India (e.g. Rao 1978; Rao and Ganapati 1966; Salvini-Plawen and Rao 1973; Sugumaran and Padmasai 2019; Sugumaran et al. 2009), but also from Panama (Calder and Kirkendale 2005), Brazil (Garraffoni et al. 2017; Jörger et al. 2014) and the Caribbean (Hochberg et al. 2014; Kånneby et al. 2014).
The morphology of all Halammohydra species is simple, yet displays special adaptations to the interstitial system. It is thought to be derived from a medusa that has lost its umbrella (Remane 1927). The entire body is ciliated and the gastric tube is thus likely homologous to the manubrium of a hydromedusa. It is connected to the aboral cone (reduced umbrella) via a neck. Two whorls of tentacles and one whorl of statocysts are attached to the aboral cone and there is an adhesive organ for temporary fixation at the tip of it. Due to its flexible body and the cilia, the animal is perfectly adapted for moving in the interstitium. Similar to other meiofaunal groups, Halammohydra has a direct development without a pelagic larva. The absence of a polyp stage and the morphology of statocysts led Remane (1927) to place Halammohydra among the Trachylinae, and further into Narcomedusae because of similarities between their larval stages. With the discovery of Otohydra Swedmark & Teissier, 1958, another fully ciliated meiofaunal medusa with direct development, the order Actinulida with the two families Halammohydridae and Otohydridae was erected (Swedmark and Teissier 1958, 1959). For a long time, the exact position within the cnidarian tree was not known for sure, until Collins et al. (2008) presented a phylogenetic study of Trachylinae, with a special focus on the placement of Halammohydra. Before the publication of Collins et al., no molecular data were available for this genus. The results confirmed their placement in Trachylinae and a closer relationship to the family Rhopalonematidae (Trachymedusae). There are no sequences available for Otohydra, so the validity of the taxon Actinulida cannot be investigated. The organization of the entire body of Halammohydra is described by histology (Remane 1927; Swedmark and Teissier 1967) and immunohistochemistry (Polte and Schmidt-Rhaesa 2011), and specific structures by ultrastructure (Clausen 1991, 2000; Ehlers 1993). Clausen did an ultrastructural study on nematocysts in the entire body (Clausen 1991) and on microsporidia in the adhesive organ of Halammohydra intermedia Clausen, 1967 (Clausen 2000), and Ehlers investigated the male gonad ultrastucturally, which he defined as a gonadal compartment in the gastrodermis (Ehlers 1993). These are the only ultrastructural studies. Therefore, there is a need for a more detailed examination to do potential phylogenetic considerations.
This insufficient morphological knowledge needs to be updated and summarized to answer general questions about the systematic position of the group and function of certain body structures. Especially a closer look at the adhesive organ and its function, as well as the female reproduction system is needed, as there is presently only one study on the male gonadal compartment (Ehlers 1993). All this information combined is essential to validate the systematic position within the Trachylinae and can help to clarify the relation to the genus Otohydra. In the present work, we present an ultrastructural study of the entire body of Halammohydra vermiformis Swedmark & Teissier, 1957. It can be identified by its conical adhesive cone and the 7 (3 aboral and 4 subaboral) and sometimes 8 (4 and 4) tentacles. Aboral tentacles are slender and shorter than subaboral ones, whereas subaboral tentacles are unequal in length. They have a variety of body shapes from elongated to round and a cnidom consisting of stenoteles and isorhizas. With this study, we add information to the published findings, especially on details of the gonadal structure and the aboral cone.
Material and methods
Specimens of Halammohydra vermiformis were collected in the south of Sylt (Hörnum, 54°45.352 N, 8°17.666 E, two males) and on Helgoland in Germany (at the sandy island called “Dune”, 54°11.394 N, 7°54.723 E, one female). In Hörnum, sediment samples of coarse sand were taken with a shovel from the beach at low tide and processed at the Wadden Sea Station in List (Alfred Wegener Institute, AWI). At Helgoland, sediment samples were collected in the shallow subtidal in 50 cm water depth at the North shore of the island “Dune” and processed at the Biological Institute Helgoland (AWI). Samples were stored in a cool environment and covered by a few centimeters of water, not longer than 1 week.
For extraction, a 7% magnesium chloride solution was used to anesthetize the animals. A small portion of the sand was mixed with this solution and incubated for 10–15 min with occasional stirring. The supernatant of the sample was decanted into a sieve with a 63 µm mesh size and the animals were collected under a stereomicroscope. Individuals of Halammohydra vermiformis were examined further under the compound microscope with higher magnification (Leica DM2500) and documented by photos and videos with a handycam (Sony). Numerous specimens were investigated within the frame of another project and of the 46 specimens of H. vermiformis, three mature specimens were selected for transmission electron microscope (TEM) investigation. Two males and one female were relaxed with magnesium chloride and fixed in Trumps (combination of sodium cacodylate buffer, formalin, and glutaraldehyde).
Specimens were postfixed with osmium tetroxide (1%, in sodium-cacodylate buffer) and embedded in LR White resin following a modified protocol by McDonald (1984) and Purschke et al. (1991). A combination of semi-thin (0.5 µm) and ultrathin (70 nm) sections was used to section through the entire animals. Ultrathin sections were contrasted with lead citrate and uranyl acetate and investigated using a Zeiss EM902A TEM. Digital photos were taken. Semi-thin sections were stained with toluidine blue and investigated with light microscopy. Slides were scanned automatically with a light microscope (LEICA DM6000B) and corresponding software (LEICA MetaMorph 1.5.0). Acquired images were adjusted using ImageJ (version 1.52a), Adobe Photoshop and Adobe Illustrator. During the preparation process, one male was accidentally destroyed from the center of the gastric tube to the mouth opening.
Results
Habitus of Halammohydra vermiformis
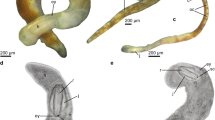
Specimens of Halammohydra vermiformis are composed of an elongated gastric tube or manubrium and an aboral cone, bearing one whorl of statocysts and two whorls of tentacles (aboral and subaboral, Fig. 1A–D). On one end of the gastric tube is the mouth opening with the short and slightly tapering proboscis (Fig. 1E) and long cilia sticking out of the opening (Fig. 1F). In living animals, the short proboscis makes irregular circular movements. Aborally, the gastric tube is connected to the cone via a neck, characterized by its smaller width (Fig. 1D). The gastrodermis is of darker color and extends from right above the proboscis to the neck. In the neck, the yellow to brown color slowly fades into transparency, which is similar to the rest of the body. The lighter structure in the gastric tube is the gonadal compartment. Due to its extensive volume, it shifts the gastrodermis and thus the mouth opening to one side. Depending on the state of contraction, the neck is rather long and thin and connects to the conical-shaped aboral cone (Fig. 1D). In addition, the transition from the gastric tube into the neck is gradual and only visible by the narrowing of the overall diameter. On the oral end of the cone, one whorl of four statocysts is attached, followed by two whorls (subaboral and aboral) of four tentacles each (as in the investigated males, in total eight tentacles) or three aboral and four subaboral tentacles (as in the investigated female, seven tentacles in total). Tentacles of both rings alternate in position to each other. The adhesive organ has a shape of an inverted cone and is located at the tip of the aboral cone (Fig. 1D). All tentacles are of the same slender shape, without any indents along their length or conspicuous diameter change at the bases. Tentacles of the aboral whorl are of the same length and visibly shorter than the majority of the subaboral whorl (Fig. 1C). One tentacle of the subaboral whorl is at least two times longer than the others (Fig. 1B). The entire body is covered with cilia, which are used for gliding. For this, all tentacles are directed orally and the animal glides with the aboral cone in front (Fig. 1C). All investigated specimens were moving very actively and rarely adhering to sediment particles or the petri dish.
Light microscopy images of the habitus of Halammohydra vermiformis (male). A It has an elongated gastric tube (gt) with a lighter-colored gonadal compartment (g) and a terminal mouth opening (mo) connected to an aboral cone (ac) via a neck. Two whorls of tentacles, aboral (ab) and subaboral (sub) are connected to it. B One tentacle of the subaboral whorl is about two times longer (arrowhead). C When gliding, all tentacles show in the oral direction. D Magnification of the aboral cone, showing the statocysts (st) and the adhesive organ (arrowhead). E Magnification of the mouth opening with its small proboscis (arrowheads). F Further magnification showing the long mouth cilia (arrowheads) sticking out of the mouth
The gastric tube
Epidermis and mesoglea
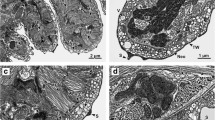
The general ultrastructure of specimens of H. vermiformis consists of an epidermis and a gastrodermis separated by a layer of extracellular matrix called mesoglea (Fig. 2A). A single layer of epitheliomuscular cells (EMCs) surrounds the entire body (Fig. 2B). This cell type has a more or less columnar shape in cross section and is attached basally to the mesoglea. In some areas, cells connect to the mesoglea along almost their entire width. Other areas have a higher amount of cell sections basally. Apically, most cells have a slightly increased width forming the outer surface. Cell nuclei are mainly in a central position and show uncondensed chromatin with a slightly visible nucleolus. Electron dense, round vesicles (Fig. 2C) with a diameter of roughly 0.3 µm occur close to the apical membrane and the entire outer surface is covered by an irregular-shaped electron-dense layer. Apically, electron-dense sections of the membranes connecting each cell are adherence junctions (Fig. 2C). The entire body is covered with cilia; in all observed cases, the cells were monociliary. In the basal part of the cells, clusters of longitudinally oriented myofibrils and more cellular compartments are present per section than apically (Fig. 2B, D, E), which shows basally branching cells, that interdigitate to a high degree. The resulting basal compartments are mostly filled with myofibrils. In general, the mesoglea is thin and homogeneous. In the oral part of the gastric tube, it has a thickness of about 0.3 µm with a few variations (Fig. 2B). Aborally, the thickness increases up to 0.9 µm with high variation (Fig. 2E). It has a more irregular shape and strong connections to basal myofibrils of the EMCs, indicated by electron-dense spots, likely hemidesmosomes, at the boundary between the layers (Fig. 2D, E).
Semi-thin section (A) and ultrastructural images (B–G) of the gastrodermis and epidermis in the gastric tube of Halammohydra vermiformis. A Overview of a section through the gastric tube close to the mouth opening showing the gastrodermis (ga) and gonadal compartment (g) surrounded by an epidermis (e), separated by a thin layer of mesoglea (arrowhead), which seems to vanish on one side (arrows). B Epidermal cells have more or less central nuclei (n) and basal myofibrils (mf) close to the mesoglea (me), which are especially concentrated in the basal cell sections (arrowheads). C Magnification of the apical part of the epithelium, with apical electron-dense vesicles (arrowheads), adhesive junctions (aj), cilia (ci) and a thin irregular-shaped electron-dense layer covering the epidermis (arrow). D Aboral part of the gastric tube with several nematoblasts (nb) and electron-dense structures (arrowheads) connecting basal extensions. E In the neck, strong connections of the epidermis to the mesoglea are indicated by electron-dense spots (arrowheads). F Orally located gastrodermal cells (of A) have basal nuclei (n) and cilia (ci) close to the surface. Some cells contain apical vesicles (arrowhead). G Aborally, the gastrodermis consists of lighter digestive cells (dc) with big electron lucent (v) and smaller electron-dense vesicles (arrowhead), and club-shaped gland cells (gc) with round secretory vesicles (sv) and cisternae of rough endoplasmic reticulum (rER)
Throughout the body, the epidermis undergoes slight changes in the shape of cells, the density of muscle fibers, and the extent of basal branching. Orally, the thickness of the epithelium varies between 8 and 15 µm. The cells are rather wide and extend mostly throughout the entire height of the epithelium (Fig. 2B). Only a few basal extensions interrupt this pattern and the density of the myofibrils is low. The thickness of the epithelium changes only slightly in the aboral direction. There is a slight increase of thickness in an area orally, close to the mouth opening, and a stronger increase aborally (up to 29 µm, Fig. 2D), right below the transition into the neck. These two locations are areas of nematocyst development, which can be recognized by several inserted cells and developing nematocysts (= nematoblasts). The aboral region of nematocyst development is larger and only on one side of the body (the gastrodermal side). Only a few nematocysts were documented in other parts of the epidermis. Further in the aboral direction, the number of basal cell compartments and the density of the myofibrils increases. In contrast to the epidermis of the mouth opening, basal myofibrils are more concentrated. Additionally, the mesoglea is less homogenous and varies in thickness. The basal region of the epithelium consists of several slender cells and some of them extend apically (Fig. 2D). Others are only located basally. In the neck region (Fig. 2E), the basal extensions dominate half of the epidermis and are filled completely with muscle fibers. Electron dense thickened regions of the membrane, putative adherens junctions, are present between the basal cell compartments (Fig. 2D, E). The thickness of the muscular basal part of the epithelium increases from about 2.3 µm (mouth opening) to 7.5 µm (neck) with some variations. The general thickness of the epithelium decreases from up to 29 µm, at the aboral end of the gastric tube, to 11 to 13.5 µm in the neck.
Gastrodermis
Mesoglea separates the epidermis from the gastrodermis and mostly also the gonadal compartment (Fig. 2A). The gastrodermis is an epithelium of mostly columnar cells, which extend from the gastral lumen to the mesoglea or gonadal compartment. There are different types of cells visible throughout the gastric tube, which are described from oral to aboral in the following.
At the mouth opening, columnar cells are filled completely with several small inclusions, apical electron-dense vesicles and a basal nucleus (Fig. 2A, F), and apically cilia insert into the cell, which sticks out of the mouth opening (Fig. 1F). Another cell type is of the same size, but with fewer inclusions and no apical vesicles (Fig. 2F). The regions with these two cell types overlap partly (Fig. 2A, F) and are limited to the area of the mouth opening. Both cell types form an epithelium with an almost smooth surface to the lumen and cilia run parallel to the cell surface (Fig. 2F). The main cell type of the gastric tube is a digestive type (Fig. 2G). Digestive cells extend from the gastral lumen to the mesoglea with varying width, where the widest part is basal. The cytoplasm is clear without many inclusions and some large vesicles are present (Fig. 2G). Apically, the cell is filled with several small inclusions and throughout the cell, there are large electron-dense irregular shaped vesicles (Fig. 2G). In between the digestive cells, there are gland cells, less in abundance than the digestive cells and not spanning the entire height of the epithelium (Fig. 2G). Gland cells are filled with many small inclusions and larger round secretory vesicles. These vesicles are electron dense and have a regular shape with a smooth surface. Basally, the cell is filled with cisternae of the rough endoplasmic reticulum.
The entire gastric tube of the living animal is very flexible and changes the overall diameter and the diameter of the lumen frequently. In the aboral direction, however, the lumen decreases until it reaches the neck, where only some cilia fit into the lumen until it closes completely. The digestive cell type continues into the oral part of the neck, where a different cell type and orientation are present. Cells, which were directed to the center of the gastric tube are now without orientation. Additionally, there are no large electron-dense irregular-shaped vesicles and gland cells are lacking completely (Fig. 5B).
Gonadal compartment—male
The other tissue present in the gastric tube is the gonadal compartment which takes up a large amount of space in the gastric tube (Figs. 1A, 3A). It extends from right above the mouth opening to under the transition into the neck and shifts the entire gastric system to one side. Like the gastrodermis, it is over most of its extent separated from the epidermis by a thin layer of mesoglea (Fig. 3B). Gastrodermis and gonadal compartment are not separated by mesoglea. On the gonadal side of the gastric tube, the mesoglea ends abruptly on both sides of the gonadal compartment (Figs. 2A, 3C) and germ cells bulge towards the epidermis and restrict it to a thin layer. This was noticed in many sections throughout the gastric tube, extending from the start of the gonadal compartment at the mouth opening until the transition into the neck, where the mesoglea is completely closed again. In semi-thin sections of another specimen is a visible indent in a short section of the gastric tube at the exact position of the lacking mesoglea (Fig. 3A). The same was observed in the female specimen (Fig. 4E, E’; see below). Unfortunately, the ultrastructurally investigated individual was destroyed in the central region of the gastric tube to the mouth, so there is no ultrastructural image of this region in a male specimen.
Semi-thin (A) and ultrastructural images (B–E) of a male gonadal compartment of Halammohydra vermiformis. A Cross-section of the center of the gastric tube with the gonadal compartment (g), gastrodermis (ga) and epidermis (e). There is an indent on the gonadal side (arrowhead). B Image of all tissues in the gastric tube, the mesoglea (me) separating epidermis (e) from the gonadal compartment (g) and gastrodermis (ga) and a nucleus (n) of a germ cell. C The mesoglea (me) ends abruptly on both sides of the indent (arrowhead). D In between germ cells, groups of cilia (ci) and developing spermatozoa (sp) are visible. E Magnification of a spermatozoon with a nucleus (n) of condensed chromatin and two mitochondria (mi). The cilium is not in the plain of this section
Female gonadal compartment of Halammohydra vermiformis with oocytes from aboral to oral. A Light-microscopy image of the female gastric tube with mouth opening (mo). Arrowheads indicate three oocytes in the gonadal compartment. Arrows indicate the position of the sections shown in B–G. B–G Semi-thin sections of the gastric tube. C’–G’ Ultrastructural images according to C–G. B Aboral part of the gastric tube with epidermis (e), gastrodermis (ga) and a thickening due to the gonadal compartment (g), (C) which increases in size in oral direction. C’ As in the male, the mesoglea (me) ends abruptly (arrowhead), but the germ cells are closely packed with a big uniform nucleus (n) and prominent nucleolus (nu). D Both aborally located, smaller oocytes (oo) have the same structure of a prominent nucleus (n) surrounded by yolk (y). D’ The yolk is filled completely with different types of vesicles. E In the center of the gastric tube, there is a strong indentation (arrowhead). E’ In this area, the epidermis loses its well-ordered character and there is no clean separation of epidermal (e) and germ cells (g). F The orally located oocyte has three different areas and a nucleus. The yolk (y) is enclosing a lighter structure (ls) orally, which is connected to a darker structure (ds) surrounding the nucleus (n). F’ Magnification of the lighter and darker structure with groups of smooth endoplasmic reticulum (arrowhead, as in the lighter structure). There is no membrane separating both structures visible. G In the oral direction, the lighter structure disappears. G’ An uneven membrane with extensions (arrowheads) separates the yolk from other structures
Cells of the male gonadal compartment are loosely connected with no prominent electron-dense cell connections (Fig. 3B–D). Some cells are in close contact with adjacent cells (Fig. 3C), while others have small (Fig. 3B) to large (Fig. 3D) gaps separating them. The cells themselves are filled completely with electron-dense structures and a large nucleus, which shows uncondensed chromatin and a prominent nucleolus. In the extracellular area, there are several groups of cilia visible (Fig. 3D). Throughout the entire gonadal compartment, no specialized areas were documented. Cells with uncondensed nuclei are next to patches of cilia and between them are different spermatogenesis stages. The putatively mature spermatozoon is round-headed; it has a nucleus with condensed chromatin and below this two mitochondria, which are round and almost equally sized (Fig. 3E). The attachment of cilia or basal bodies, as well as the apical vesicles, were not observed in the sections investigated.
Gonadal compartment—female
The female gonadal compartment starts one-third of the gastric tube above the mouth opening and extends up to the transition into the neck (Fig. 4A). It varies in shape depending on the volume of the oocytes in different areas of the gonadal compartment. The specimen used for closer examination had three voluminous oocytes, with two smaller ones (aboral) and one bigger one (oral), which are even visible in light microscopy (Fig. 4A). Aborally, smaller and immature germ cells are located and create a thickening on one side of the gastric tube (Fig. 4B). It increases in oral direction and is separated from the epidermis by mesoglea (Fig. 4C). As in the male specimens, there is an abrupt ending of the mesoglea on both sides of the gonadal compartment (Fig. 4C’), but there is always a thin layer of epidermal cells separating the gonadal compartment from the outer environment. The germ cells themselves are densely packed and have a nucleus with a prominent nucleolus. In the oral direction, two oocytes are located. Both have the same structure with a large portion of yolk and a central nucleus (Fig. 4D). The oocytes take up almost half of the gonadal compartment, which also is filled with additional germ cells. In this section, the gastrodermis is shifted to one side and takes up one-third of the gastric tube. Ultrastructural images show, that the yolk is filled up with darker structures, many electron-dense and some less dense vesicles (Fig. 4D’). The nucleus has a very prominent nucleolus, as the other germ cells. In the center of the body, there is an indent into the side of the gonadal compartment (Fig. 4A, E). It is more distinctly visible than in the male (compare to Fig. 3A). Ultrastructural images show a complete deformation of the epidermis and a loss of the single-layered character (Fig. 4E’). The cells have different shapes and some single germ cells intrude into the epidermis. This indent is clearly visible in light microscopy as well and was documented in other specimens too (Fig. 4A).
The third and largest oocyte consists of three substructures and the nucleus. Aborally, the oocyte has the yolk wrapping around an electron-lucent structure (here called lighter structure), which is connected to an electron-dense structure (here called darker structure) on one side. The darker structure encloses the nucleus (Fig. 4F). There is no clear separation visible between the lighter and the darker structure (Fig. 4F’). The lighter structure is completely filled with vesicles and smooth endoplasmic reticulum. In contrast, the darker structure consists of many granules and occasional groups of vesicles and smooth endoplasmic reticulum. Orally, the lighter structure vanishes and the yolk takes up most of the space (Fig. 4G). It is separated from the darker structure and all other cells by a plasma membrane, which also produces extensions reaching into the darker structure (Fig. 4G’). The darker structure with the nucleus does not proceed in the oral direction. There was no nucleus found in the yolk. This third oocyte takes up almost the entire gonadal compartment. There are only a few undeveloped germ cells left on the gastrodermal side.
The aboral cone
Statocysts and tentacles
The transition from the gastric tube into the neck is gradual, so there is no clear definition of the start of the neck. Only the decrease in diameter indicates the beginning of the neck region (Fig. 5A), which then transitions into the aboral cone. The aboral cone starts with the connection of four statocysts alternating with the subaboral tentacles (Fig. 5C). Statocysts are connected via a thin stalk of epidermal cells (Fig. 5E). The statocysts themselves consist of a few epidermal cells and a vacuole (Fig. 5E, F). There was no statolith visible, as well as, no destruction of tissue was documented in the semi- or ultra-thin sections. A dark line, likely mesoglea, runs within the connection of the statocysts to the aboral cone, but it is only visible in the semi-thin section (Fig. 5E). Next to the connection to the aboral cone, cilia with a strongly developed rootlet are located (Fig. 5F). In the living animal, the statocysts show a pulsating movement.
Semi-thin section (A, C, E) and ultrastructural images (B, D, F, G) of the orally directed part of the aboral cone, statocysts and tentacle of Halammohydra vermiformis. A At the transition of the neck into the aboral cone the gastrodermis (ga) has a closed lumen and myofibrils (mf) create a light ring structure. First statocysts (st) are visible. B Ultrastructure of A showing the ring structure of the myofibrils (mf), the gastrodermis (ga) and the irregular-shaped mesoglea (me) with several electron-dense spots (arrowheads). C Orally directed part of the aboral cone with a strong ring of myofibrils (mf) surrounding the gastrodermis; statocysts (st) and subaboral tentacles (sub) connecting to the cone. D Ultrastructure of C showing a strong muscle ring. E Statocysts are connected to the cone via a thin link of epidermal cells. There is a dark line running through the connection (arrowhead). The position of the statolith is indicated by an empty vacuole (v). F Ultrastructure of a statocyst (st) with its vacuole (v), a cilium (ci) and cilia rootlet (cr) at its connection to the cone. G Transverse section of a tentacle with an outer epidermis (e) with central nuclei (n) and basal myofibrils (mf) and an inner tentacle tissue filled almost completely with a vacuole (va). At the outer edge, myofibrils (mf) are visible and in the center, a nucleus (n) surrounded by a rough endoplasmic reticulum (rER) is located. Tentacles are covered in cilia (ci)
Aboral tentacles insert alternating to subaboral ones and thus have the same orientation as the statocysts. All tentacles are solid, made up of epidermis surrounding a chordoid rod of inner cells. Both layers are separated by a thin and uniform mesoglea. The central cells are voluminous and elongated, per cross section the rod is composed of only one cell (Fig. 5G). These cells have almost no inclusions, but contain a single vacuole, displacing the cytoplasm to the outer edge and around the nucleus (Fig. 5G), which is evenly electron-lucent colored and has a prominent nucleolus. It is surrounded by cisternae of rough endoplasmic reticulum and a few smaller vesicles. In the periphery, close to the mesoglea, few myofibrils with circular orientation are present. Nuclei of the epidermal cells are located in the center. Tentacles are covered with cilia.
Muscle and nerve ring
Longitudinally oriented myofibrils are located in the basal part of the epidermis. In the gastric tube, the area filled by myofibrils is thin orally (Fig. 2B) and increases in the aboral direction until they fill almost all basal cell sections (Fig. 2D), which continues and intensifies in the aboral cone (Fig. 5A–D) and results in a pronounced ring structure. In the orally directed part of the aboral cone, the emerging ring is uneven and has a thickness of about 5–12 µm. The wide apical cells displace the slender basal cells, which have a flame-like shape (Fig. 2E). This continues aborally until the slender cells almost dominate the epithelium (Fig. 5B). The ring becomes more pronounced and even in thickness until it reaches its maximum of 10.5 to 13.4 µm when the gastrodermis has its minimum of 10.5 µm and subaboral tentacles connect to the aboral cone (Fig. 5C, D). Throughout this process, the layer of mesoglea thickens slightly and gets more irregular. Some parts of it extend in between the basal epidermal cells and there are strong cell connections visible as electron-dense spots (Fig. 5B). Aborally, the muscle ring regresses and the basal cells containing muscle fibers get wider (Fig. 6A, B). The layer of mesoglea evens out.
Semi-thin section (A, E) and ultrastructural images (B-D, F, G) of the central part of the aboral cone of Halammohydra vermiformis. A In the center of the aboral cone, the ring of myofibrils (mf) around the gastrodermis (ga) regresses and the nerve ring (nr) emerges. B Ultrastructure of A. The mesoglea (me) is less irregular and the nerve ring (nr) consists of several small cells. C, D Magnifications of B showing dense cored synapses (C) in the gastrodermis (arrowheads) and (D) in the nerve ring (arrowheads). E Aborally, the inner tentacle tissues (tt) of the subaboral tentacles connects to the gastrodermis (ga). Aboral tentacles (ab) are not connected to the cone here. F, G Gastrodermis (ga) and inner tentacle tissues (tt) are separated by mesoglea (me) but there are connections between (F) the inner tentacle tissues (arrowheads) and (G) between tentacle tissue and gastrodermis (arrowhead)
This is the area where the nerve ring emerges (Fig. 6A–D). Several small cell sections accumulate circular around the regressing muscle ring and the gastrodermis. These cell sections are neurites and vary in size and shape and show no nucleus. Throughout this ring, there are several small, electron-dense structures, which are dense cored synapses (Fig. 6D). These round vesicles contain an electron-dense spot and are distributed in the entire ring with varying densities. Even in the gastrodermis, a cluster of these structures was documented (Fig. 6C). Throughout the body, no such accumulation was noticed.
When the nerve ring regresses, the myofibrils emerge again but enclose the inner tentacle tissue, which is now in contact with the gastrodermis (Fig. 6E). A layer of mesoglea separates both tissue types. Occasionally, the core cells of adjacent tentacles have contact with each other (Fig. 6F) and with the gastrodermis (Fig. 6G) by very small connection sites. Surrounding myofibrils run into the tentacles (Fig. 7A) and the concentration decreases around the adhesive organ (Fig. 7B).
Ultrastructural images of the adhesive organ (ao) of Halammohydra vermiformis from oral to aboral. A Orally, the inner tentacle tissues (tt) deform the shape of the adhesive organ (ao). It is filled with electron-dense vesicles (arrowheads). In the center a small lumen with cilia (ci) is visible. All tissues are separated by mesoglea (me) and surrounded by myofibrils (mf). B Aborally, the adhesive organ is round in shape and completely filled with electron-dense vesicles (arrowheads). The lumen increases and more cilia (ci) are visible. There is no mesoglea surrounding the structure but a thin layer of myofibrils (mf). C Magnification of the lumen (l) filled with cilia (ci). D Magnification of the edge with myofibrils (mf)
Adhesive organ
The adhesive organ begins in the center of the aboral cone, where the inner tentacle tissue of both whorls connects to the gastrodermis (Fig. 7A). Orally it is diamond-shaped because of the contact with the inner tentacle tissues and aborally it is round with 20 µm in diameter (Fig. 7B). It consists of a few cells with poorly visible cell borders. The cells are mainly filled with electron-dense round vesicles with smooth surfaces. In the center of the adhesive organ is a lumen (Fig. 7A–C), which increases in diameter in the aboral direction. The lumen is completely filled with cilia, which insert into the cells apically and stick out of the aboral pore creating the aboral cilia tuft (Fig. 7C). The orally directed part of the adhesive organ is enclosed with mesoglea, which is not visible in the aborally directed part anymore. Instead, there is a structure of plasma membranes creating a pronounced boarder separating cells of the adhesive organ from other epidermal cells around it (Fig. 7D). Some myofibrils are visible as lines around the adhesive organ.
Cnidome
Throughout the entire body, different densities of nematocysts are visible. All nematocysts and developmental stages were found in the epidermis and none in the gastrodermis. Additionally, three specialized sections of developing nematocysts were documented. The first and smallest is located on the gastric tube in the oral direction. This location contains only a low density of developing nematocysts. The second location on the gastric tube is in the aboral direction, close to the transition into the neck on the gastrodermal side. Here, the epidermis is noticeably thicker than in the rest of the gastric tube (Fig. 2D). The third and largest location is at the basal part of the tentacles (Fig. 8A). In this region, the density of developing nematocysts is highest and there is a slight thickening of the epidermis compared to other locations in the tentacle, but this thickening does not result in a prominent bulb at the base when observed in light microscopy (Fig. 1A–D).
A–D Different developmental stages of nematocysts in Halammohydra vermiformis. A Longitudinal section of a tentacle within an area of nematocyst development showing the epidermis (e) with nematocysts (nc) in nematoblasts (nb), mesoglea (me) and inner tentacles tissue (tt). B Cross section of two nematoblasts with developing nematocysts (nc). The nematocysts have a wide electron-lucent capsular wall (cw) and a dense core matrix (ma). The cell itself contains a large nucleus (n) with the nucleolus, is filled with a prominent rough endoplasmic reticulum (rER) and sections of the external tubes (et) are visible. C. Longitudinal section of a nematoblast with developing elongated nematocysts. It is differentiated in a capsular region (cr) and an external tube (et). D Developmental stage before maturity with visible spines (sp) and a thick capsular wall (cw). E–H Ultrastructural images of heteronemes. E Section of a developing and mature heteroneme. In the developing nematocyst, the capsule wall (cw) is still wide and the crescent-shaped nucleus (n) touches the capsule. The shaft (sh) is visible in the center of the nematocysts. In the mature heteroneme, the three-bladed structure of the tubule (tu) is visible. F Longitudinal section of a mature heteroneme with spines (sp) and stylets (sty) on the shaft, the inverted capsule membrane (im) and the outer capsule wall (cw). G Basal cross section of a developing heteroneme showing a striated pattern on the inverted capsule membrane (arrowhead). H Apical cross section of a heteroneme with a folded inverted capsule membrane (im) enclosing the three stylets (sty). I–K Second type of nematocyst. I Overview of the capsule with a ring of microtubules (mt) surrounding the structure. J Magnification of the nematocyst with a thick tubule (tu) and microtubules (mt). K In some parts of the epidermis, only the groups of microtubules (arrowheads) are visible
Different developmental stages occur next to each other in these regions. Early stages of the nematoblast, the cell developing into the nematocyst, contains a voluminous nucleus with uncondensed chromatin and a prominent nucleolus, a well-developed rough endoplasmic reticulum and the developing nematocyst (Fig. 8B). The nematocyst has an electron-lucent capsular wall and a dense matrix. Throughout the cytoplasm of the cell, sections of the external tube coiling are located (Fig. 8B, C). In a longitudinal section, the division in the capsular region, determined by the capsular wall, and the external tube is clearly visible (Fig. 8C). In a later stage, there is no external tube, the composition of the matrix is granular and the prominent shaft of the heteroneme is visible (Fig. 8D). The nematocyst now fills the entire cell but the capsular wall is still wide and the nucleus has a crescent shape, touching the capsule (Fig. 8E).
One of the most abundant types of nematocysts in this individual is the heteroneme (Fig. 8E–H). It occurs on the entire body, but with different densities. On the gastric tube is a minor occurrence, except for the two regions of development. On the aboral cone is a higher abundance. Most of the heteronemes are on the tentacles. Here, they occur in higher density but without a specific pattern or concentration of some cysts. Heteronemes have an oval shape with a round basal and a slightly tapering apical part. An operculum is located apically and directed to the outer environment. Its prominent shaft with spines and stylets easily recognizes this type of nematocyst. Even in an immature stage, it is visible (Fig. 8D, E). The shaft itself is enclosed in the inverted sac of the capsular membrane. There are three stylets connected to the shaft. In a cross section of the apical part, the three stylets are visibly enclosed by a folded inverted capsular membrane (Fig. 8H). When the nematocyst is cut in the basal region, no stylets are visible, but a striated pattern on three bulges connected to the inverted capsular membrane (Fig. 8G). The tube itself coils in the capsule and has a three-bladed shape in cross section (Fig. 8E). Besides heteronemes, there is a second type of nematocysts. It has a lower abundance and was only documented on the tentacles (Fig. 8I–K). There is no shaft but a thick tubule enclosed in the capsule. A ring of microtubules, visible as electron-dense circles, surrounds the nematocyst itself (Fig. 8J). In some cells, only a group of microtubules is visible, indicating the capsule in a different section of the cell (Fig. 8K).
Discussion
Halammohydra vermiformis is the most suitable species within its genus to investigate the internal structure due to its high abundance and the low number of tentacles since a higher number might obscure the overall picture. The combination of ultrathin and semi-thin sections is ideal to section specimens in a reasonable time, but it includes the danger to miss ultrastructural data when a structure is accidentally present only in semi-thin sections. In our case, this happened for e.g. the connection of the statocysts to the aboral cone. All observed statocysts lacked a statolith, but as there was no obvious destruction in the tissue of this area, we assume that the statoliths dissolved during the preparation. Additionally, it was difficult to investigate the tentacles as a whole, because they spread out too far to get sections along their entire length. Therefore, structural changes, e.g. densities of nematocysts, could not be followed reliably along the tentacle length.
Hörnum on Sylt is a reliable location for finding specimens of Halammohydra. Due to the difficulty to identify species in this genus in general, the exact species composition at this location is not known with certainty. Previously, specimens found in Hörnum were identified as H. octopodides Remane, 1927 (Polte and Schmidt-Rhaesa 2011), but our investigations of the morphology identified specimens from this location clearly as H. vermiformis.
In general, H. vermiformis has the typical structure of a cnidarian, with the outer epidermis, inner gastrodermis and a layer of mesoglea separating them. Cells of the epidermis have cilia, which are used for gliding between the sand grains. This is a clear adaptation to the interstitial system (Giere 2009), as pelagic medusae do not use them for locomotion (Werner 1964). The surfaces of the epidermis are covered by an irregular-shaped electron-dense layer, which is likely the glycocalyx. The chordoid structure of the inner tentacle tissue with voluminous vacuoles is very common among Hydrozoa and functions as a hydrostatic skeleton (Thomas and Edwards 1991). Together with the mesoglea and the musculature formed by epitheliomuscular cells (EMCs) in the tentacle, the hydrostatic skeleton is crucial for the structural integrity and tentacle movement. The mesoglea is mostly thin throughout the body, except for the location of the muscle ring in the aboral neck. It is irregular shaped, due to the tension of the surrounded muscles and its needs to be thicker for the myofibrils to anchor (Haynes et al. 1968), as this is a highly flexible part. Myofibrils of the neck insert into the tentacles and enable a high movability of them.
Next to the muscle ring, there is a prominent nerve ring in the aboral cone. This was already shown with immunohistochemical stainings by Polte and Schmidt-Rhaesa (2011) in H. octopodides. Apart from this dominant nerve ring, other neural structures documented by Polte and Schmidt-Rhaesa (2011) such as tentacular neurites, the mouth cone plexus or the oral nerve ring were not observed in this study, mainly because it is impossible with a combined ultrathin/ semi-thin sectioning to reliably follow the course of fine neurites. A connection of the nerve ring to the statocysts was not observed as well, but since this is a sensory organ, we expect a connection. When the medusa changes its position, the free statocysts move and come in contact with the surrounded sensory cilia to detect the movement (Singla 1975).
A higher concentration of neurites and myofibrils indicates controllability and thus a better ability to move and change shape. This was documented for the mouth opening. It moves in circular motions and can also be stretched greatly for food intake of bigger prey. Moreover, the long cilia of the gastrodermis might help to transport the food into the mouth for further digestion. There are different cell types of the gastrodermis involved in this process, which were already described by Remane (1927) and categorized as cell types a to h. Depending on the different concentrations of the respective cell types, he defined five zones throughout the body. In this study, not all of the described cell types were observed. At the mouth opening, two cell types are present, which might represent zone I (cell type a and b) of Remane’s description. The lighter cell type might be mucus cells, which release mucus for protection and the darker cell type might be secretive cells, which initiate the extracellular digestion of food (Thomas and Edwards 1991). This cannot be said with certainty, as this is no histochemical study, but the position of these cells and the unusual placement of the cilia close to the cell surface support this assumption. The second zone of Remane’s classification was not documented in this study, but cell types of the third zone are present. One of the major parts of the gastrodermis is the typical cnidarian digestive EMC (Remane 1927; Thomas and Edwards 1991). Scattered in between are the characteristic gastric zymogen or gland cells (Haynes et al. 1968; Remane 1927). They release enzymes into the gastric lumen and initiate extracellular digestion. Predigested food particles can then be ingested into the digestive EMC for further digestion (Haynes et al. 1968). As a result of ingested food, the gastrodermis gains coloration, which is visible even in light microscopy. In the neck, the resorption of food particles reduces or stops due to the small or absent lumen. Furthermore, a different shape and orientation of the cells occurs because of the reduction of the diameter and the loss of the lumen. This corresponds to Remane’s zone IV (cell type g, Remane 1927). Remane also described a fifth zone (cell type h), which is actually the aboral adhesive organ. It does not belong to the gastrodermis but is a result of an invagination of the ectoderm early in development (Swedmark and Teissier 1957). The gastrodermis itself persists as a chordoid rod into the tentacles.
Next to the gastrodermis, the gonadal compartment is located in the gastric tube as well. There is only one study regarding the male reproductive system (Ehlers 1993) and none for the female one. The exact position of the gonadal compartment was described before as being between the epidermis and the gastrodermis of the gastric tube (Remane 1927; Swedmark and Teissier 1966), until Ehlers (1993) regarded it to be part of the gastrodermis. The same is assumed here, because mesoglea surrounds both, the gastrodermis and the gonadal compartment, except for the gap, where no mesoglea could be detected. It was described before that the spermatozoa and oocytes are released into the water via the gastrodermis and through the mouth opening (Clausen 1971; Ehlers 1993; Swedmark and Teissier 1966). This study showed a slight indent on the side of the gonadal compartment of the male, which is very pronounced in the female. It is likely that the spermatozoans and oocytes are not released through the gastric system but by a rupture of the epidermis. This would explain the disruption of the single-cell epithelium in the epidermis at this location. Spawning through a rupture of the epidermis is also very common in Hydrozoa (Thomas and Edwards 1991). As a meiofaunal animal, it is essential to have an effective reproduction process. Many meiofaunal organisms have an internal fertilization or stick the oocytes to a sand grain (Giere 2009), which was described for Halammohydra (Swedmark and Teissier 1957). It stays attached until the end of the embryonal development. Since observations of fertilization are missing, it remains unclear how this is coordinated.
Structures of the male gonadal compartment and of the spermatozoa are similar to the ones described by Ehlers (1993), with the exception of the acrosome and cilia, as they were not observed directly here. Only the groups of cilia between the germ cells show their presence. The female gonadal compartment of Halammohydra has not been described so far. It is possible that there is a stratification of maturation from aboral (youngest) to oral (mature) because of the different positions of the germ cells, immature oocytes and the mature oocyte. The orally located oocyte has a more complex structure than the other two. There are two distinct regions, the lighter and darker structure with nucleus, and the yolk portion. Both are separated by what seems to be a membrane, which appears to be quite unusual in this position. A composition of the mature egg by the oocyte and a separate yolk cell seems unlikely, as the yolk compartment does not include a nucleus and because such a separation is not present in earlier stages of oogenesis. Therefore, the function of this intra-oocyte membrane must remain unexplained. Similar structures have not been reported, to our knowledge (see e.g. Beams and Kessel 1983; Tardent 1984). The prominent indent in the center of the female body might be a leftover of a recent rupture of the epidermis after a release of an oocyte. However, if oocytes are arranged in a maturity gradient, the position of the indent would indicate that not the most mature oocyte, but the second one was released. This needs further investigation to clarify if this is a typical process or just a random finding.
In the studied individual, the adhesive organ consists of large cells with many secretory vesicles and a central lumen filled with cilia. The temporary adhesion process might be a combination of the production of secretion and action of the myofibrils surrounding the structure. Central cells produce an adhesive secretion and the cilia transport it to the tip of the aboral cone. Additionally, the adhesive organ might have a slight sucker effect for temporary adhesion. Surrounding myofibrils can help to release the individual by contraction. As H. vermiformis is mostly seen freely swimming and less sedentary compared to other species, the adhesive organ might differ from more adhesive species, like H. schulzei Remane, 1927, in terms of thickness of the myofibril layer or the overall size and shape of the adhesive organ. In this study’s individual, the adhesive organ was conical shaped. Other species have cup- (H. octopodides, H. intermedia Clausen, 1967, H. adherens Swedmark & Teissier, 1967) or even pear shaped (H. schulzei) adhesive organs, which reach deeper into the aboral cone and thus have a thicker layer of secretory cells (Clausen 1967). Some authors described the adhesive organ as part of the gastrodermis (Remane 1927) or with a cup of gastrodermal cells and mesoglea around it (Swedmark and Teissier 1966). This could not be verified here, because we did not observe a connection of the gastrodermis and the cells of the adhesive organ. Moreover, a layer of mesoglea surrounding the structure was only documented on the most oral part. Compared to other species, the adhesive organ of H. vermiformis is small and less sunken in the aboral cone. In H. schulzei and H. octopodides, for example, the adhesive organ is rather deep and therefore the connection with the mesoglea and gastrodermal cells creating a cup is possible. Remane (1927) documented this connection but interpreted it as part of the gastrodermis. These examples show the importance of investigations into the adhesive organ of additional species, to find functional and thus potential species-specific differences.
Halammohydra vermiformis was described to have two types of nematocysts: stenoteles and isorhizas (Clausen 1967; Swedmark and Teissier 1957). Stenoteles were found on the entire body, whereas isorhizas are only present on the tentacles (Clausen 1967). This distribution could more or less be confirmed in this study. Heteronemes were seen on the entire surface of the animal, with increased concentration in certain regions, like the developing areas for nematocysts or on the tentacles. Since only two types of nematocysts were described for this species, the observed heteronemes are potential stenoteles. The second type present was only documented on the tentacles, but the exact type of nematocysts could not be revealed with certainty. The lack of a shaft is a character of a haploneme type (Östman 2000) and the ring of supportive rods was documented in a study about the nematocysts in H. intermedia before (Clausen 1991). Further identification cannot be done using our images, but it is very likely, that these nematocysts are isorhizas, as they have been documented for this species.
Within the cnidarian tree, Halammohydra was placed into Hydrozoa and Trachylinae very early for obvious reasons (Remane 1927; Swedmark and Teissier 1957): The absence of a polyp stage and the structure of the statocysts are clear characters of Trachylinae. A further placement was problematic for a long time, but due to possession of stenoteles, direct development and placement of the gonad (here referred to as gonadal compartment) on the manubrium (here referred to as gastric tube) favored the relation close to Trachymedusae and Narcomedusae (Bouillon and Boero 2000a; Clausen 1967; Marques and Collins 2004; Remane 1927). A molecular study by Collins et al. (2008) confirmed these suggested relations and revealed a possible origin of Halammohydra within Rhopalonematidae (Trachymedusae). Only the structure of the statocysts supports this origin (Bouillon and Boero 2000b; Bouillon et al. 2006). The position of the gonadal compartment differs from the typical Hydrozoa, where it is located in the epidermis. Here it is located in the gastrodermis but has a connection to the epidermis through a gap in the mesoglea. This intermediate stage is unusual. The position in the gastrodermis is uncommon but was documented for other hydrozoan species as well (Bouillon et al. 2004).
With the discovery of Otohydra, another meiofaunal cnidarian, the order Actinulida was created for Otohydra and Halammohydra because of the fully ciliated body, the same type of statocysts and the lack of a planula stage (Swedmark and Teissier 1958, 1966). Despite these similarities, there are great differences as well. Most prominent is the lack of an adhesive organ and the presence of an umbrella in Otohydra. Additionally, the genus only has one whorl of tentacles and is hermaphroditic (Clausen 1971). There are no ultrastructural or molecular data available on Otohydra, so this has to be examined further to verify the relation to Halammohydra and the existence of the order Actinulida.
In summary, the ultrastructural investigation of H. vermiformis helped to understand the general organization of this genus and filled some gaps, especially concerning the gonadal compartment. Further studies should be done on the adhesive organ with different species because this might reveal the exact process of adhering and help to better correlate morphology to behavioral differences and thus environmental preferences. Additionally, an ultrastructural and molecular study of Otohydra is needed to finally answer the questions regarding the composition and arrangement of genera within the family Actinulida and their placement in the Cnidarian tree.
References
Beams HW, Kessel RG (1983) Cnidaria. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates. John Wiley & Sons, New York, pp 31–66
Boaden P (1960) Three new gastrotrichs from the Swedish west coast. Cah Biol Mar 1:397–406
Boaden P (1961) Littoral interstitial species from Anglesey representing three families new to Britain. Nature 191:512
Boaden P (1963) The interstitial fauna of some North Wales beaches. J Mar Biol Assoc UK 43:79–96. https://doi.org/10.1017/S0025315400005270
Boaden P (1966) Interstitial fauna from northern Ireland. Veröff Inst Meeresforsch Bremerhaven 2:125–136
Bouillon J, Boero F (2000a) The Hydrozoa: a new classification in the light of old knowledge. Thalassia Salent 24:3–45. https://doi.org/10.1285/i15910725v24p3
Bouillon J, Boero F (2000b) Synopsis of the families and genera of the Hydromedusae of the world, with a list of the worldwide species. Thalassia Salent 24:47–296
Bouillon J, Medel MD, Pagès F, Gili JM, Boero F, Gravili C (2004) Fauna of the mediterranean Hydrozoa. Sci Mar 68:5–438
Bouillon J, Gravili C, Gili JM, Boero F (2006) An introduction to Hydrozoa. Publ Scientifiques Du Mus Paris 194:104–121
Calder DR, Kirkendale L (2005) Hydroids (Cnidaria, Hydrozoa) from shallow-water environments along the Caribbean coast of Panama. Caribb J Sci 41:476–491
Clausen C (1963) The hydrozoan Halammohydra found in Norway. Sarsia 11:17–20. https://doi.org/10.1080/00364827.1963.10410280
Clausen C (1967) Morphological studies of Halammohydra Remane (Hydrozoa). Sarsia 29:349–370. https://doi.org/10.1080/00364827.1967.10411094
Clausen C (1971) Interstitial Cnidaria: present status of their systematics and ecology. Smithson Contrib Zool 76:1–8
Clausen C (1991) Differentiation and ultrastructure of nematocysts in Halammohydra intermedia (Hydrozoa, Cnidaria). Coelenterate biology: recent research on Cnidaria and Ctenophora. Springer, Cham, pp 623–628
Clausen C (2000) Light and ultrastructural observations on a microsporidium in the hydrozoan Halammohydra intermedia (Cnidaria). Sarsia 85:177–180. https://doi.org/10.1080/00364827.2000.10414568
Collins AG, Bentlage B, Lindner A, Lindsay D, Haddock SHD, Jarms G, Norenburg JL, Jankowski T, Cartwright P (2008) Phylogenetics of Trachylina (Cnidaria: Hydrozoa) with new insights on the evolution of some problematical taxa. J Mar Biol Assoc UK 88:1673–1685. https://doi.org/10.1017/S0025315408001732
Dahl E (1953) The Narcomedusa Halammohydra octopodides Remane new to Sweden. Kungl Fysiografiska Sällskapets I Lund Förhandlingar 22:1–2
Ehlers U (1993) Ultrastructure of the spermatozoa of Halammohydra schulzei (Cnidaria, Hydrozoa): the significance of acrosomal structures for the systematization of the Eumetazoa. Microfauna Mar 8:115–130
Garraffoni AR, Di Domenico M, Hochberg R (2017) New records of marine Gastrotricha from São Sebastião Island (Brazil) and the description of a new species. Mar Biodivers 47:451–459. https://doi.org/10.1007/s12526-016-0486-1
Giere O (2009) Meiobenthology: The microscopic motile fauna of aquatic sediments. Springer, Heidelberg
Greeff R (1870) Protohydra leuckarti. Eine marine Stammfrom der Colenteraten. Z Wiss Zool 20:37–54
Haynes JF, Burnett AL, Davis LE (1968) Histological and ultrastructural study of the muscular and nervous systems in Hydra. I. the muscular system and the mesoglea. J Exp Zool 167:283–293. https://doi.org/10.1002/jez.1401670304
Hochberg R, Atherton S, Kieneke A (2014) Marine Gastrotricha of Little Cayman Island with the description of one new species and an initial assessment of meiofaunal diversity. Mar Biodivers 44:89–113. https://doi.org/10.1007/s12526-013-0186-z
Jörger KM, Stoschek T, Migotto AE, Haszprunar G, Neusser TP (2014) 3D-microanatomy of the mesopsammic Pseudovermis salamandrops Marcus, 1953 from Brazil (Nudibranchia, Gastropoda). Mar Biodivers 44:327–341. https://doi.org/10.1007/s12526-014-0224-5
Kånneby T, Atherton S, Hochberg R (2014) Two new species of Musellifer (Gastrotricha: Chaetonotida) from Florida and Tobago and the systematic placement of the genus within Paucitubulatina. Mar Biol Res 10:983–995. https://doi.org/10.1080/17451000.2013.872797
Kikinger R, Salvini-Plawen L (1995) Development from polyp to stauromedusa in Stylocoronella (Cnidaria: Scyphozoa). J Mar Biol Assoc UK 75:899–912. https://doi.org/10.1017/S0025315400038236
Marques AC, Collins AG (2004) Cladistic analysis of Medusozoa and cnidarian evolution. Invertebr Biol 123:23–42. https://doi.org/10.1111/j.1744-7410.2004.tb00139.x
Martínez A, Palmero AM, Del Carmen BM, Núñez J, Worsaae K (2009) Anchialine fauna of the Corona lava tube (Lanzarote, Canary Islands): diversity, endemism and distribution. Mar Biodivers 39:169–182. https://doi.org/10.1007/s12526-009-0023-6
Martínez A, Di Domenico M, Leasi F, Curini-Galletti M, Todaro MA, Zotto MD, Gobert S, Artois T, Norenburg J, Jörger KM, Núñez J, Fontaneto D, Worsaae K (2019) Patterns of diversity and endemism of soft-bodied meiofauna in an oceanic island, Lanzarote, Canary Islands. Mar Biodivers 49:2033–2055. https://doi.org/10.1007/s12526-019-01007-0
McDonald K (1984) Osmium ferricyanide fixation improves microfilament preservation and membrane visualization in a variety of animal cell types. J Ultrastruct Res 86:107–118. https://doi.org/10.1016/S0022-5320(84)80051-9
Östman C (2000) A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Sci Mar 64:31–46. https://doi.org/10.3989/scimar.2000.64s131
Polte S, Schmidt-Rhaesa A (2011) Immunohistochemical investigations of the interstitial cnidarian Halammohydra octopodides (Hydrozoa). Meiofauna Marina 19:17–32
Purschke G, Hagens M, Westheide W (1991) Ultrahistopathology of enchytraeid oligochaetes (Annelida) after exposure to pesticides—a means of identification of sublethal effects? Comp Biochem Physiol Part C Toxicol Pharmacol 100:119–122. https://doi.org/10.1016/0742-8413(91)90136-H
Rao GC (1978) On a new species of Halammohydra (Actinulida, Hydrozoa) from Andamans, India. Bull Zool Surv India 1:147–149
Rao GC, Ganapati P (1966) A report on the occurrence of an aberrant cnidarian Halammohydra octopodides Remane, in Indian waters. Curr Sci 35:129–130
Remane A (1927) Halammohydra, ein eigenartiges Hydrozoon der Nord-und Ostsee. Z Morphol Oekol 7:643–677
Salvini-Plawen L (1991) Pseudovermis thompsoni new species (Nudibranchia: Aeolidoidea) from the Northern Adriatic sea. J Moll Stud 57:179–187. https://doi.org/10.1093/mollus/57.Supplement_Part_4.179
Salvini-Plawen L, Rao GC (1973) On three new mesopsammobiotic representatives from the Bay of Bengal: species of Anthohydra gen. nov. (Hydrozoa) and of Pseudovermis (Gastropoda). Z Morphol Oekol 74:231–240. https://doi.org/10.1007/BF00375786
Schmidt P (1969) Die quantitative Verteilung und Populationsdynamik des Mesopsammons am Gezeiten-Sandstrand der Nordsee-Insel Sylt II. Quantitative Verteilung und Populationsdynamik einzelner Arten. Int Rev Hydrobiol Hydrogr 54:95–174. https://doi.org/10.1002/iroh.19690540104
Schmidt-Rhaesa A, Pyataeva S, Collins AG (2020) Cnidaria. In: Schmidt-Rhaesa A (ed) Guide to the identification of marine meiofauna. Pfeil-Verlag, München, pp 33–43
Singla C (1975) Statocysts of hydromedusae. Cell Tissue Res 158:391–407
Sugumaran J, Padmasai R (2019) Meiofaunal diversity and density of Manamelkudi – an intertidal sandy beach of Palk Bay, India. Res J Life Sci Bioinf Pharm Chem Sci 5:31–46. https://doi.org/10.26479/2019.0502.03
Sugumaran J, Naveed M, Altaff K (2009) Diversity of meiofauna of Chennai, East coast of India. Aquat Biol 24:31–34
Swedmark B, Teissier G (1957) Halammohydra vermiformis n. sp. et la famille des Halammohydridae Remane. Bull Soc Zool Fr 82:38–49
Swedmark B, Teissier G (1958) Otohydra vagans n. g., n. sp., hydrozoaire des sables apparenté aux Halammohydridées. C R Acad Sci 247:238–240
Swedmark B, Teissier G (1959) Halammohydra et Otohydra, hydrozoaires de la microfaune des sables et l’ordre des Actinulides. Proc XV Int Congr Zool 4:330–332
Swedmark B, Teissier G (1966) The Actinulida and their evolutionary significance. In: Rees WJ (ed) The Cnidaria and their evolution. Academic Press, London, pp 119–133
Swedmark B, Teissier G (1967) Structure et adaptation dHalammohydra adherens. Cah Biol Mar 8:63–74
Tardent P (1984) The differentiation of germ cells in Cnidaria. In: Halvorson HO, Monroy A (eds) The origin and evolution of sex, Alan R. Liss Inc, New York, pp 163–198
Teissier G (1950) Notes sur quelques hydrozoaires de Roscoff. Arch Zool Exp Gen 87:1–10
Thomas MB, Edwards NC (1991) Cnidaria: Hydrozoa. In: Harrison FW, Westfall JA (eds) Microscopic anatomy of invertebrates, volume 2: Placozoa, Porifera, Cnidaria, and Ctenophora. Wiley-Liss, New York, pp 91–183
Tödter L, Schmidt-Rhaesa A (2021) First record of Halammohydra (Cnidaria, Hydrozoa) on the Azores. Acoreana Special Volume 11:97–102
Werner B (1964) Halammohydra Remane, Medusennatur und Stellung im System. Verh D Zool Ges 1964:163–178
Wolff W, Sandee A, Stegenga H (1974) Halammohydra vermiformis and H. coronata (Hydrozoa), new to the fauna of the Netherlands. Neth J Sea Res 8:407–409. https://doi.org/10.1016/0077-7579(74)90008-8
Acknowledgements
We thank Sabine Gaude for the preparation and sectioning of the samples and Dr. Frank Friedrich for the help with the transmission electron microscope. We also like to thank Dr. Ilka Sötje and Dr. Sabine Holst for their helpful comments and Boris Osadchenko for their insight into his data. This study was supported by grant SCHM 1278/18-1, awarded to Andreas Schmidt-Rhaesa by the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded by Deutsche Forschungsgemeinschaft (DFG) with the grant number SCHM 1278/18-1 and conducted at the University of Hamburg and the Leibniz Institute for the Analysis of Biodiversity Change (LIB) in Hamburg (former CeNak) as part of a PhD project. No approval from research ethics committees was required to accomplish the goals of this study since experimental work was conducted on an unregulated invertebrate species.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tödter, L., Schmidt-Rhaesa, A. Ultrastructural organization of Halammohydra vermiformis Swedmark & Teissier, 1957 (Cnidaria: Hydrozoa). Zoomorphology 141, 133–149 (2022). https://doi.org/10.1007/s00435-022-00560-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-022-00560-w