Abstract
Purpose
Uterine serous carcinoma (USC) is a highly aggressive and frequently recurring subtype of endometrial cancer with limited treatment options for advanced or recurrent stages. Sulindac, a classic non-steroidal anti-inflammatory drug, has demonstrated anti-tumor activity in several pre-clinical tumor models. This study aims to evaluate the effect of sulindac on cell proliferation and invasion in USC cells.
Methods
Human USC cell lines ARK-1 and SPEC2 were treated with different concentrations of sulindac. Cell proliferation was assessed using MTT and colony formation assays. ELISA assays measured cellular stress, cleaved caspase 3 activity, antioxidant ability, and adhesion. Cell cycle arrest was evaluated by Cellometer. The invasive capability was detected by wound healing assay. Western blotting was used to analyze the changes in protein expression induced by sulindac.
Results
Exposure to sulindac decreased cellular viability in a dose-dependent manner in ARK-1 and SPEC2 cells. Sulindac effectively inhibited cell cycle progression, increased cellular stress, caused apoptosis, and reduced cell adhesion and invasion in USC cells. Additionally, sulindac decreased the expression of COX-2 and blocked phosphorylation of NF-κB induced by TNF-α.
Conclusion
Sulindac is a potential therapeutic agent for USC that deserves further exploration in pre-clinical studies and potentially future clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries, with a steadily rising incidence and mortality attributed to the global obesity epidemic and the aging population (Crosbie et al. 2022; Siegel et al. 2023). Endometrioid adenocarcinoma is the predominant histological subtype of EC, accounting for about 85–90% of cases. It is generally associated with a lower risk for progression and has a favorable prognosis when diagnosed in its early stages (Crosbie et al. 2022). Uterine serous carcinoma (USC) is the most representative non-endometrioid adenocarcinoma type, making up around 5–10% of all cases of EC, yet contributing to almost 40% of deaths related to EC (Bogani et al. 2021). Despite usually being diagnosed early, patients with USC often face a higher risk of recurrence due to its aggressive pathological features, resulting in a relatively low survival rate of 35–50% (Bogani et al. 2021; Hamilton et al. 2006). Thus, it is crucial to develop innovative treatment strategies for patients with advanced or recurrent USC to improve survival.
Chronic inflammation creates an environment that facilitates the development and progression of certain cancers (Diakos et al. 2014). The cyclooxygenase-2/prostaglandin E2 (COX-2/PGE2) pathway is considered to be a central inflammatory pathway involved in the regulation of carcinogenesis, angiogenesis, invasion, apoptosis, and resistance to chemotherapy and radiotherapy in gynecological cancers (Hashemi Goradel et al. 2019; Ye et al. 2020). Sulindac, a classic non-selective non-steroidal anti-inflammatory drug (NSAID), has been widely used for many years to treat acute or chronic inflammation by inhibiting the COX-2/PGE2 pathway and exhibits beneficial effects in the chemoprevention of colorectal, supported by animal studies, clinical trials, and epidemiological investigations (Burke et al. 2020; Madka et al. 2023; Mohammed, Yarla, Madka, & Rao, 2018). Relative to chemoprevention, sulindac also demonstrates anti-proliferative and anti-invasive capabilities that are dependent or independent of the COX-2/PGE2 pathway in multiple pre-clinical cancer models (Borneman et al. 2022; Hossain et al. 2023; Wang, Huang, Lin, Chang, & Huang, 2022). The combination of sulindac and chemotherapeutic agents or immunoreagents produces synergic effects on cell proliferation and tumor growth in cancer cells and mouse models of cancer, including lung cancer, leukemia, triple-negative breast cancer, and head and neck cancer (Gross et al. 2014; Hossain et al. 2023; Kim et al. 2015; Moon & Lerner 2002).
A recent meta-analysis showed that overexpression of COX-2 is significantly associated with more advanced clinical stage, myometrial invasion, lymph node metastasis, and poor prognosis in EC (M. Li et al. 2020). Long term use of aspirin, one of the most common NSAIDs, significantly reduced the risk of EC and is hypothesized to be via its effects on inhibition of the COX-2/PGE2 pathway (Takiuchi et al. 2018). The epidemiological evidence suggested that COX-2 may be a potential therapeutic target for EC. Compared with normal endometrial tissues, overexpression of COX-2 has also been observed in USC tissues (Lyndin et al. 2022). Considering the aggressive clinical features of USC, in the current study, we aimed to investigate the effect of sulindac on cell proliferation, apoptosis, cell cycle, invasion, and cellular stress in USC cells, as to provide pre-clinical evidence for the use of sulindac in the prevention and adjuvant treatment of EC.
Materials and methods
Cell culture and reagents
The human serous endometrial cancer cell lines, ARK-1 and SPEC2, were used in this study. The ARK-1 cells were provided by Dr Santin (Yale University School of Medicine) and were cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific; Waltham, MA). The SPEC2 cells were provided by Dr Kauffman (University of North Carolina at Chapel Hill) and were maintained in DMEM/F12 medium supplemented with 10% FBS. All media contained 1% penicillin/streptomycin and 1% L-Glutamine (Gibco Cell Culture, CA). Both cell lines were cultures in humidified 5% CO2 at 37 °C. The cell lines are authenticated annually by LabCorp (Burlington, NC) using short tandem repeat (STR) profiling. Sulindac sulfide was purchased from MedChemExpress (Newark, NJ). For the tumor necrosis factor-alpha (TNF-α)-related studies, the cells were cultured in RPMI-1640 and DMEM/F12 medium supplemented with 0.5% charcoal-stripped FBS (Thermo Fisher Scientific). All the antibodies were obtained from Cell Signaling Technology (Beverly, MA) and ABclonal (Woburn, MA). The Western Lightning™ Plus Chemiluminescence Reagent was purchased from PerkinElmer (Waltham, MA), Inc. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
MTT assay
The ARK-1 and SPEC2 cells were seeded at 4000–8000 cells/well in 96-well plates and incubated for 24 h at 37 °C. The cells were subsequently treated with varying concentrations of sulindac (from 1 to 250 μM) for 72 h. 5 μl of MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solution (5 mg/ml) was added to each well, and the plates were incubated at 37 ˚C for 1 h. The cells were lysed with 100 μl dimethyl sulfoxide (DMSO) per well, and absorbance was measured at 562 nm using a microplate reader (Tecan; Morrisville, NC). The effect of sulindac on cell proliferation was assessed as a percentage of control, and IC50 was calculated by the AAT Bioquest calculator (Sunnyvale, CA).
Colony assay
The ARK-1 and SPEC2 cells were plated overnight in 6-well plates at a density of 300 cells/well and 400 cells/well, respectively, and then were treated with sulindac (25, 75, and 100 μM) for 72 h. The cells were then cultured for 12–14 days, and the medium was changed every three days. The colonies were stained with 0.5% crystal violet and counted under a microscope.
Cleaved caspase-3 assay
The ARK-1 and SPEC2 cells were cultured at a concentration of 2 × 105/wells in 6-well plates overnight, and then treated with sulindac (25, 75, and 100 μM) for 14 h. After being washed twice with PBS, cell lysates were collected with 150 µl/well 1X caspase lysis buffer. The BCA assay (Thermo Fisher Scientific) was used to measure the concentration of protein in the lysis buffer. Lysates (30–40 µg) were mixed and incubated with reaction buffer containing 200 µM caspase 3 substrates (AAT Bioquest) for 30 min in black, clear bottom, 96-well plates. The fluorescence intensity of cleaved caspase 3 was measured at Ex/Em 341/441 nm by a Tecan microplate reader.
Reactive oxygen species (ROS) assay
The quantification of intracellular ROS was measured by 2',7'-Dichlorodihydrofluorescein diacetate (DCFH-DA, Sigma-Aldrich) assay. The ARK-1 and SPEC2 cells were seeded at 8000 and 12,000 cells/well in 96-well plates overnight, respectively, and then treated with sulindac (25, 75, and 100 μM) for 6—8 h at 37 °C. 15 µM DCFH-CA in phenol red-free medium was added to each well for 30 min. The fluorescence intensity was measured at Ex/Em 485/525 nm using a Tecan microplate reader.
JC-1 assay
Mitochondrial membrane capacity was monitored using a JC-1 (Sigma-Aldrich) molecular probe. The ARK-1 and SPEC2 cells at 1 × 104 and 2.5 × 104 cells/well were cultured in 96-well plates overnight and treated with sulindac (25, 75, and 100 μM) for 4–6 h. Following the treatment, the cells were incubated with 200 µM JC-1 dye for 30 min at 37 °C. The green JC-1 signal was detected at wavelengths Ex/Em 485/535 nm, and the red JC-1 signal was measured at Ex/Em 535/590 nm using a Tecan microplate reader.
TMRE assay
The ARK-1 and SPEC2 cells were seeded at 0.5 × 105 and 1.5 × 105 cells/well in black clear-bottom, 96-well plates overnight, respectively. The cells were treated with sulindac (25, 75, and 100 μM) for 4–6 h and then incubated with 1 mM Tetramethylrhodamine Ethyl Ester (TMRE, Sigma-Aldrich) for another 30 min at 37 °C. The plate was washed twice with PBS and measured at Ex/Em 549/575 nm using a Tecan microplate reader.
Trolox equivalent antioxidant capacity (TEAC) assay
The antioxidant capacity of ARK-1 and SPEC2 cells was measured using a total antioxidant capacity (TEAC) assay. The cells were seeded for 24 h in 6-well plates at a density of 2–3 × 105 cells/well, and then were treated with sulindac (25, 75, and 100 μM) and incubated at 37 °C overnight. The cell supernatants were collected using cold PBS after centrifuging at 12,000 rpm for 15 min at 4 °C. The protein concentration in the supernatant was determined using BCA assay. After adjusting the sample volumes with cold PBS, the equal amount and volume of samples were added to 96-well plates. A volume of 150 μl ABTS solution (Sigma-Aldrich) was added to each well and mixed thoroughly. The plates were incubated for 5 min on an orbital shaker at room temperature, and optical density (OD) was read at 405 nm using a Tecan microplate reader.
Cell cycle assay
The ARK-1 and SPEC2 cells were plated in 6-well plates at a concentration of 2–3 × 105 cells/well overnight and treated with sulindac (25, 75, and 100 μM) for 24 h at 37 °C. Then the cells were harvested from the plates using 0.25% Trypsin (Sigma-Aldrich) and fixed in a 90% methanol solution for 1 h. The cells were resuspended in a solution containing propidium iodide (PI), RNase, and Triton X-100 for 30 min. The cell cycle progression of all samples was measured by Cellometer (Nexcelom, Lawrence, MA, USA) and analyzed using FCS4 Express software (Molecular Devices, Sunnyvale, CA, USA).
Adhesion assay
After 96-well plates were coated with 80 μl laminin-1 (10 μg/ml) (Sigma-Aldrich) and incubated overnight at 4 °C, the blocking buffer (0.5% BSA in PBS) was added to each well and incubated for 30 min. The ARK-1 and SPEC2 cells were added at 1–2 × 105/well along with sulindac (25, 75, and 100 μM) and incubated at 37 °C for 90 min. 100 μl of 5% glutaraldehyde was added to each well, and then the plates were incubated for 30 min at room temperature. The plates were stained with 0.1% crystal violet (100 µl/well) for 15 min at room temperature after washing with PBS buffer. The dye was then solubilized via the addition of 100 µl of 10% acetic acid to each well which dissolves the dye. The plates were measured at 575 nm using a Tecan microplate reader.
Wound healing assay
The capacity for migration in ARK-1 and SPEC2 was evaluated using wound healing assay. The ARK-1 and SPEC2 cells were seeded in 6-well plates at a density of 5 × 105/well and cultured until they reached > 80% confluency. After changing the culture medium to 1% FBS, the uniform wounds were created by scratching a line across the cell monolayer using a 200 μl pipette tip. The cells were washed twice with PBS and treated with sulindac (5, 10, and 25 μM) for 24–48 h. Photos of each well were taken 24 and 48 h after treatment. The width between the scratch was measured and calculated using the ImageJ software (National Institutes of Health; Bethesda, MD).
Western blotting
The ARK-1 and SPEC2 cells were cultured at a concentration of 2.5–4 × 105 cells/well in 6-well plated overnight. The cells were then treated with sulindac (25, 75, and 100 μM) for 8–24 h. Total protein was extracted with RIPA lysis buffer, and the protein concentration in the supernatants was determined by BCA assay (Thermo Fisher Scientific). Equal amounts of protein were separated by 10% or 12% SDS-PAGE and transferred to PVDF membranes (Bio-Rad; Hercules, CA). After blocking with 5% fat-free milk for 1 h at room temperature, the membranes were then incubated with primary antibodies overnight on an orbital shaker at 4 °C. The next day, the membranes were washed and incubated with secondary antibodies for 1 h at room temperature. Immunoblotting signals were developed using an enhanced electrochemiluminescence kit on the ChemiDoc Image System (Bio-Rad). The quantitative assessment of protein expression was analyzed by ImageJ software (National Institutes of Health; Bethesda, MD).
Statistical analysis
All experiments were performed in triplicate to ensure consistency of results. Data were reported as mean ± SD. GraphPad Prism 8 was used to determine the statistical differences between groups. Student's t-test or one-way ANOVA was used to analyze statistical significance. p < 0.05 was considered statistically significant.
Results
Sulindac inhibited cell proliferation in USC cell lines
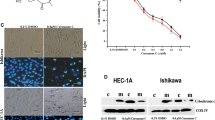
The ARK-1 and SPEC2 were exposed to sulindac at concentrations varying from 1 to 250 μM for 72 h, and the inhibition of cell proliferation was detected by MTT assay. MTT results demonstrated that with increasing concentrations of sulindac, cell viability decreased in both cell lines. The mean IC50 values of sulindac were 86.06 μM in ARK-1 cells and 74.66 μM in SPEC2 cells, respectively (Fig. 1A and supplemental Figure). The long-term effects of sulindac on proliferation were also evaluated by colony formation in USC cell lines. The ARK-1 and SPEC2 cells were treated with 25, 75, and 100 μM for 72 h, followed by subsequent culture of the cells for 12–14 days. Sulindac at 100 μM significantly decreased colony-forming capacity of both ARK-1 and SPEC2 cells by 76.0% and 57.2% compared with untreated cells, respectively (Fig. 1B).
Sulindac inhibited cell proliferation in USC cells. The ARK-1 and SPEC2 cells were treated with sulindac (1 to 250 μM) for 72 h. MTT results showed that sulindac inhibited cell proliferation in a dose-dependent manner in both cell lines (A). The ARK-1 and SPEC-2 cells were treated with sulindac at 25, 75, and 100 μM for 72 h, and then cultured for an additional 12–14 days. Colony assays found that sulindac effectively inhibited colony formation in the ARK-1 and SPEC2 cells (B). Western blotting results showed that sulindac inhibited the expression of COX-2 after treatment with sulindac for 12 h in both cell lines (C). ARK-1 cells were pre-treated with sulindac at 75 μM for 2 h and then treated with 10 ng/ml TNF-α for 15 and 30 min. Western blotting results indicated that pre-treatment with sulindac significantly reversed TNF-α-induced upregulation of phosphorylation of NF-κB (D). *p < 0.05, **p < 0.01 compared with control. #indicates comparison between groups. #p < 0.05
To investigate whether inflammatory pathways were involved with the inhibition of cell proliferation by sulindac in USC cells, the ARK-1 and SPEC2 cells were treated with sulindac (25, 75, and 100 μM) for 12 h, and Western blotting results showed that sulindac reduced the expression of COX-2 in both cell lines (Fig. 1C). Furthermore, the ARK-1 cells were pre-treated with sulindac at a dose of 75 μM for 2 h, followed by treatment with 10 ng/ml of TNF-α for 15 and 30 min. Western blotting demonstrated that pre-treatment with sulindac significantly reversed TNF-α-induced upregulation of phospho-NF-κB in the ARK-1 cells (Fig. 1D). All these results suggest that the inhibitory effect of sulindac on cell proliferation may be dependent on the COX-2 pathway.
Sulindac induced cellular stress in USC cell lines
To investigate the effect of sulindac on cellular stress, the ARK-1 and SPEC2 cells were treated with sulindac (25, 75, and 100 μM) for 6–8 h. DCFH-DA assay showed that sulindac treatment significantly increased ROS production in a dose-dependent manner in both cell lines. Treatment of both cells with 100 μM sulindac showed a 1.29-fold increase in the level of ROS in ARK-1 cells and a 1.19-fold increase in SPEC2 cells compared with control cells (Fig. 2A). To further characterize the effect of sulindac on mitochondrial function, the JC-1 and TMRE assays were used to detect alterations of mitochondrial membrane potential after 4–6 h of sulindac exposure. Both assays showed that sulindac reduced mitochondrial membrane potential in the ARK-1 and SPEC2 cells at doses of 75 and 100 μM. Sulindac at 100 μM significantly reduced JC-1 levels by 29.8% and TMRE levels by 17.5% in the ARK-1 cells, and by 24.1% and 17.0% in SPEC2 cells, compared with untreated cells (Fig. 2A). The TEAC assay was used to detect the total antioxidant capacity of ARK-1 and SPEC2 cells after treatment with sulindac overnight. The results showed that sulindac at 100 μM significantly reduced the antioxidant capacity by increasing the TEAC optical density (OD) level to 25.5% in ARK-1 cells and 32.6% in SPEC2 cells (Fig. 2B). Furthermore, sulindac regulated the expression of endoplasmic reticulum (ER) stress-related proteins in both cell lines. Western blotting showed that sulindac up-regulated the protein expression of BiP, ATF-4, and PERK in the ARK-1 and SPEC2 cells after 8–12 h of treatment (Fig. 2C). These results support that sulindac exerts an antioxidant role in USC cell proliferation.
Sulindac induced cellular stress in USC cells. The ARK-1 and SPEC2 cells were treated with 25, 75, and 100 μM sulindac for 4–8 h. Sulindac at 75 and 100 μM significantly increased cellular ROS levels and decreased JC-1 and TMRE levels in both cell lines (A). Treated with sulindac at 75 and 100 μM overnight significantly reduced antioxidant capacity (B). Western blotting results revealed that treatment of sulindac for 8–12 h increased the expression of the BiP, ATF-4, and PERK proteins (C). *p < 0.05, **p < 0.01 compared with control
Sulindac inhibited cell cycle progression
To investigate whether sulindac impacts cell cycle progression of USC cells, the cell cycle profiles of the ARK-1 and SPEC2 cells after 24 h of sulindac treatment were assessed by Cellometer. Results indicated that sulindac increased the proportion of ARK-1 cells in G2/M phase and SPEC2 cells in G1 phase. Sulindac at a dose of 100 μM increased G2/M phase from 30.55% to 38.31% in the ARK-1 cells, and G1 phase in the SPEC2 cells was increased from 38.71% to 57.51% (Fig. 3A). Western blotting results demonstrated that sulindac decreased the expression level of several cell cycle-related proteins, such as CDK4, CDK6, and cyclin D1, in both cell lines after 24 h of treatment (Fig. 3B). These results suggest that the inhibition of USC cell growth by sulindac may be achieved through inhibition of cell cycle progression.
Sulindac induced cell cycle arrest and apoptosis in USC cells. The ARK-1 and SPEC2 cells were treated with 25, 75, and 100 μM sulindac for 24 h. The cell cycle profile was assessed by Cellometer. Sulindac resulted in cell cycle G2 phase arrest in the ARK-1 cells and G1 phase arrest in the SPEC2 cells (A). Western blotting results showed that sulindac inhibited the expression of CDK4, CDK6 and cyclin D1 after treatment for 24 h in both cell lines (B). *p < 0.05,**p < 0.01, compared with control
Sulindac induced apoptosis in USC cell lines
To evaluate whether sulindac-induced cell growth inhibition corresponded to apoptosis in USC cells, the levels of cleaved caspase 3 were detected by enzyme-linked immunosorbent assay (ELISA). Sulindac at 75 and 100 μM increased production of cleaved caspase 3 in the ARK-1 cells to 1.36-fold and 1.63-fold, and in SPEC2 cells to 1.21-fold and 1.26-fold, respectively, compared with untreated cells (Fig. 4A). Meanwhile, western blotting showed that treatment of cells with sulindac for 8 h decreased the expression of Bcl-2 and Mcl-1, and increased the expression of Bax in both cell lines (Fig. 4B).
Sulindac induced apoptosis in USC cells. The ARK-1 and SPEC2 cells were treated with 25, 75, and 100 μM sulindac for 14 h, and cleaved caspase 3 ELISA assay showed that 75 and 100 μM sulindac treatment significantly increased the activity of cleaved caspase 3 (A). Western blotting results showed that sulindac suppressed expression of Bcl-2 and Mcl-1 and increased the expression of Bax after treatment for 8 h in both cell lines (B). *p < 0.05, **p < 0.01, compared with control
Sulindac inhibited adhesion and invasion in USC cell lines
To investigate the effects of sulindac on adhesion and migration in USC cells, laminin-1 adhesion and wound healing assays were employed in the ARK-1 and SPEC2 cells. After treatment with sulindac for 90 min in laminin-1 coated 96 well plates, a significant decrease in cell adhesion was observed in the ARK-1 and SPEC2 cells compared with untreated cells. Sulindac at 100 μM inhibited adhesion by 21.8% in the ARK-1 cells and 18.3% in the SPEC2 cells, compared to the control cells (Fig. 5A). Additionally, the wound healing assay showed that sulindac at doses of 10 and 25 μM reduced the migratory capacity in both cell lines after 48 h of treatment. Compared with control cells, sulindac at a dose of 25 μM increased wound healing widths by 2.23-fold in ARK-1 cells and 1.89-fold in SPEC2 cells (Fig. 5B). To determine the effect of sulindac on epithelial-mesenchymal transition (EMT), the ARK-1 and SPEC2 cells were treated with sulindac for 24 h. Western blotting results showed that sulindac reduced the expression of vimentin and β-Catenin (Fig. 5C). These results indicate that sulindac inhibits metastatic potential of USC in vitro.
Sulindac inhibited adhesion and invasion in USC cells. The effects of sulindac on the adhesion and migration were investigated by a laminin-1 adhesion assay and a wound healing assay. The ARK-1 and SPEC2 cells were treated with 25, 75, and 100 μM sulindac for 90 min. The laminin-1 assay showed that 75 and 100 μM sulindac significantly inhibited cell adhesion in both cell lines (A). ARK-1 and SPEC2 cells were treated with 5, 10 and 25 μM sulindac for 48 h. The wound healing assay showed that 10 and 25 μM sulindac inhibited cell migration after 48 h of treatment in both cell lines (B). Western blotting showed that sulindac decreased the expression of vimentin and β-catenin after 24 h of treatment in both cell lines (C). *p < 0.05, **p < 0.01, compared with control
Discussion
Long-term NSAID treatment is effective in reducing inflammation in the body and lowering the risk of breast, colon, and endometrial cancers (Bakierzynska et al. 2023; Meyerhardt et al. 2021; Takiuchi et al. 2018). Given that increased inflammatory signaling activity is a key contributor to promoting carcinogenesis and progression of EC, it is necessary to explore how NSAIDs, such as sulindac, can impede cell proliferation and invasion in EC, including USC (Thrastardottir et al. 2023). In this study, we first explore the effects of sulindac on cell proliferation, cellular stress, apoptosis, cell cycle and invasion in USC cells. The results showed that sulindac effectively inhibited the expression of COX-2 and blocked TNF-α induced phosphorylation of NF-κB. Treatment USC cells with sulindac significantly reduced cell proliferation, caused cell cycle arrest, elevated cellular stress, and decreased the invasive capability in USC cells, suggesting that targeting COX-2/PGE2 signaling pathway by sulindac is an effective strategy in inhibiting cell growth in USC.
Sulindac appears to have multiple functions in inducing ROS in cancer cells, including increasing sensitivity of cancer cells to oxidative stress inducers, and protecting normal cells from oxidative stress (Allani et al. 2018; Konjalwar et al. 2024). Increased cellular ROS production by sulindac is the basis for reducing mitochondrial membrane potential, inducing cell cycle arrest and causing apoptosis in cancer cells (H. R. Kim et al. 2006; Konjalwar et al. 2024). There is conflicting evidence that the generation of ROS may be dependent or independent of COX-2-related mechanisms (Hashemi Goradel et al. 2019). When colon cancer cells were treated with sulindac for 6 h, cellular ROS levels began to increase and reached a peak at 18 h (Minami et al. 2005). Pre-treatment with N-Acetylcysteine (NAC) effectively blocked sulindac-mediated ROS generation and cell growth inhibition (Minami et al. 2005). Our results show that sulindac increased ROS levels and decreased mitochondrial membrane potential, with increased expression of cellular stress-related proteins. Notedly, at the same time, sulindac also significantly reduced total intracellular antioxidant capacity in the ARK-1 and SPEC2 cells. These results indicate that induction of oxidant stress and reduction of antioxidant capacity may be crucial in the sulindac-mediated anti-cancer activity specifically in USC cells.
The COX-2/PGE2 pathway possesses the capacity to modulate cell cycle progression, and inhibition of COX-2 can lead to cell cycle arrest in cancer cells (Halim et al. 2023). Our previous study showed that treatment of ovarian cancer cells with the COX-2 inhibitor celecoxib for 24 h significantly increased cell cycle G1 proportion (Suri et al. 2016). While there is conflicting data regarding the impact of sulindac on the cell cycle, several studies indicated that sulindac treatment successfully triggers G1 cell cycle arrest in cancer cells through various pathways, such as miR-182/FOXO3a/Cyclin G2 and RB/p21 (Jung et al. 2005; Zhao et al. 2021). In this current study, we found that treatment of ARK-1 and SPEC2 cells with sulindac for 24 h inhibited cell progression by increasing the proportion of SPEC2 cells in the G1 phase and the proportion of ARK1 cells in the G2/M phase, both of which were accompanied by a decrease in expression of CDK4, CDK6 and cyclin D1. It has been found that COX-2 inhibitor-induced cell cycle progression was associated with decreased expression of cyclin D and cyclin-dependent kinases (CDKs) in cancer cells. Inhibition of COX-2 expression may affect cell cycle progression at the G1 or G2/M phase in cancer cells through both PG-dependent and PG-independent effects(Sobolewski et al. 2010; Toyoshima et al. 2002). The association between COX-2 and cell cycle regulators such as p53 and pRB may contribute to the different responses of USC cells to sulindac during cell cycle progression, as SPEC2 has wild-type p53, whereas ARK-1 has mutant p53 (Qu et al. 2019; Vaish et al. 2014). Together, the mechanism by which sulindac inhibits cell cycle progression remains to be examined.
Sulindac possesses distinct characteristics that allow it to effectively inhibit cell proliferation and induce apoptosis of cancer cells without being toxic to normal proliferating tissues (Li et al. 2013). Overexpression of COX-2 in normal tissues and cancer cells increases Bcl-2 activity and resistance to apoptosis, all of which can be reversed by sulindac (Hashemi Goradel et al. 2019; Kern et al. 2002; Miliński et al. 2023; Sun et al. 2002). We have previously reported that celecoxib reduced the expression of COX-2, decreased cleaved caspase 3 activity and inhibited tumor growth in a mouse model of ovarian cancer under obese and lean conditions (Suri et al. 2016). Furthermore, sulindac has been found to induce mitochondrial apoptosis by reducing the expression of the Bcl-2 protein family and activating the caspase cascades in melanoma, breast, lung, and cervical cancer cells (Hwang et al. 2013; Karl et al. 2007; Miliński et al. 2023; Sui et al. 2018). Dietary sulindac (0.5 mg per day for 9–10 weeks) inhibited tumor formation and reduced intestinal COX-2 and PGE2 to baseline levels, leading to restoration of normal levels of apoptosis in transgenic mouse models of gastrointestinal adenomas (Boolbol et al. 1996). Our results confirmed that sulindac effectively induced cleaved caspase 3 activity and reduced the expression of Bcl-2 and Mcl-1 in both USC cell lines. Additional studies in USC xenograft mouse models are needed to confirm the effects of sulindac on tumor growth inhibition and induction of apoptosis in vivo as to increase the clinical relevance of this drug.
Early invasion and intra-abdominal and extrauterine metastasis are often associated with progression and recurrence in women with USC (Bogani et al. 2021). Reducing early metastasis may be an effective way to improve the prognosis of patients with USC. Since overexpression of COX-2 increases the expression of MMPs and promotes the invasion and motility of cancer cells, COX-2 is considered an inducer of invasion and angiogenesis (Hashemi Goradel et al. 2019; Hosseini et al. 2018). Inhibition of COX-2 by celecoxib effectively reduced cell invasive ability and angiogenesis of tumor tissues in ovarian cancer cells and a transgenic mouse model of ovarian cancer (Suri et al. 2016). It has been found that sulindac significantly inhibits the migration and invasion of various types of cancer cells through different mechanisms, including inhibition of protein kinase B (Akt), COX-2/matriptase, transforming growth factor beta 1(TGF-β1)-induced EMT, NF-κB, and matrix metalloproteinases (MMPs) pathways (Cha et al. 2016; Ko et al. 2017; Poursoltani et al. 2021; Vaish et al. 2014; Vaish & Sanyal 2012). In a human colon cancer xenograft mouse model, sulindac treatment significantly decreased liver metastasis though reduction of β-catenin signaling pathway (Stein et al. 2011). Consistent with these studies, our results showed that sulindac inhibited cell adhesion and migration and simultaneously reduced vimentin and β-Catenin expression in the ARK-1 and SPEC2 cells. Altogether, these data suggest that sulindac has the potential to inhibit cancer cell invasion that may involve multiple inter-related signaling pathways.
Conclusion
While sulindac has been studied for its anti-tumor activity in multiple types of cancer cells and in mouse models of cancer, the results we presented in the manuscript is the first evidence that sulindac is able to exhibit anti-proliferative and anti-invasive effects in USC. Intervention strategies targeting the COX-2/PGE2 pathway through newly developed small molecule inhibitors or sulindac derivatives with improved efficacy and limited toxicity may be promising approaches to inhibit USC cell proliferation and invasion. Our results support further investigation of the effects of sulindac on cell proliferation and tumor growth in mouse models and primary cultures of USC to determine the potential use of sulindac as an adjuvant strategy in the prevention and treatment of highly aggressive USC disease.
Data availability
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding authors, without undue reservation.
References
Allani SK, Weissbach H, Lopez Toledano MA (2018) Sulindac induces differentiation of glioblastoma stem cells making them more sensitive to oxidative stress. Neoplasma 65(3):376–388. https://doi.org/10.4149/neo_2018_170404N245
Bakierzynska M, Cullinane MC, Redmond HP, Corrigan M (2023) Prophylactic aspirin intake and breast cancer risk; a systematic review and meta-analysis of observational cohort studies. Eur J Surg Oncol 49(10):106940. https://doi.org/10.1016/j.ejso.2023.05.015
Bogani G, Ray-Coquard I, Concin N, Ngoi NYL, Morice P, Enomoto T, Monk BJ (2021) Uterine serous carcinoma. Gynecol Oncol 162(1): 226–234. https://doi.org/10.1016/j.ygyno.2021.04.029
Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Bertagnolli MM (1996) Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res, 56(11):2556–2560.
Borneman, R. M., Gavin, E., Musiyenko, A., Richter, W., Lee, K. J., Crossman, D. K., . . . da Silva, L. M. (2022). Phosphodiesterase 10A (PDE10A) as a novel target to suppress β-catenin and RAS signaling in epithelial ovarian cancer. J Ovarian Res, 15(1), 120. https://doi.org/10.1186/s13048-022-01050-9
Burke, C. A., Dekker, E., Lynch, P., Samadder, N. J., Balaguer, F., Hüneburg, R., . . . Cohen, A. (2020). Eflornithine plus Sulindac for Prevention of Progression in Familial Adenomatous Polyposis. N Engl J Med, 383(11), 1028–1039. https://doi.org/10.1056/NEJMoa1916063
Cha, B. K., Kim, Y. S., Hwang, K. E., Cho, K. H., Oh, S. H., Kim, B. R., . . . Kim, H. R. (2016). Celecoxib and sulindac inhibit TGF-β1-induced epithelial-mesenchymal transition and suppress lung cancer migration and invasion via downregulation of sirtuin 1. Oncotarget, 7(35), 57213–57227. https://doi.org/10.18632/oncotarget.11127
Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N (2022) Endometrial cancer. Lancet 399(10333):1412–1428. https://doi.org/10.1016/s0140-6736(22)00323-3
Diakos CI, Charles KA, McMillan DC, Clarke SJ (2014) Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15(11):e493-503. https://doi.org/10.1016/s1470-2045(14)70263-3
Gross ND, Bauman JE, Gooding WE, Denq W, Thomas SM, Wang L, Grandis JR (2014) Erlotinib, erlotinib-sulindac versus placebo: a randomized, double-blind, placebo-controlled window trial in operable head and neck cancer. Clin Cancer Res, 20(12), 3289–3298. https://doi.org/10.1158/1078-0432.Ccr-13-3360
Halim PA, Sharkawi SMZ, Labib MB (2023) Novel pyrazole-based COX-2 inhibitors as potential anticancer agents: Design, synthesis, cytotoxic effect against resistant cancer cells, cell cycle arrest, apoptosis induction and dual EGFR/Topo-1 inhibition. Bioorg Chem 131:106273. https://doi.org/10.1016/j.bioorg.2022.106273
Hamilton, C. A., Cheung, M. K., Osann, K., Chen, L., Teng, N. N., Longacre, T. A., . . . Chan, J. K. (2006). Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer, 94(5), 642–646. https://doi.org/10.1038/sj.bjc.6603012
Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K (2019) Cyclooxygenase-2 in cancer: A review. J Cell Physiol 234(5):5683–5699. https://doi.org/10.1002/jcp.27411
Hossain, F., Ucar, D. A., Monticone, G., Ran, Y., Majumder, S., Larter, K., . . . Miele, L. (2023). Sulindac sulfide as a non-immune suppressive γ-secretase modulator to target triple-negative breast cancer. Front Immunol, 14, 1244159. https://doi.org/10.3389/fimmu.2023.1244159
Hosseini, F., Mahdian-Shakib, A., Jadidi-Niaragh, F., Enderami, S. E., Mohammadi, H., Hemmatzadeh, M., . . . Hassannia, H. (2018). Anti-inflammatory and anti-tumor effects of α-l-guluronic acid (G2013) on cancer-related inflammation in a murine breast cancer model. Biomed Pharmacother, 98, 793–800. https://doi.org/10.1016/j.biopha.2017.12.111
Hwang, K. E., Park, C., Kwon, S. J., Kim, Y. S., Park, D. S., Lee, M. K., . . . Kim, H. R. (2013). Synergistic induction of apoptosis by sulindac and simvastatin in A549 human lung cancer cells via reactive oxygen species-dependent mitochondrial dysfunction. Int J Oncol, 43(1), 262–270. https://doi.org/10.3892/ijo.2013.1933
Jung B, Barbier V, Brickner H, Welsh J, Fotedar A, McClelland M (2005) Mechanisms of sulindac-induced apoptosis and cell cycle arrest. Cancer Lett 219(1):15–25. https://doi.org/10.1016/j.canlet.2004.06.015
Karl T, Seibert N, Stöhr M, Osswald H, Rösl F, Finzer P (2007) Sulindac induces specific degradation of the HPV oncoprotein E7 and causes growth arrest and apoptosis in cervical carcinoma cells. Cancer Lett 245(1–2):103–111. https://doi.org/10.1016/j.canlet.2005.12.034
Kern MA, Schubert D, Sahi D, Schöneweiss MM, Moll I, Haugg AM, Schirmacher P. (2002) Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology, 36(4 Pt 1), 885–894. https://doi.org/10.1053/jhep.2002.36125
Kim HR, Kim EJ, Yang SH, Jeong E. T, Park C, Kim SJ, Park R (2006) Combination treatment with arsenic trioxide and sulindac augments their apoptotic potential in lung cancer cells through activation of caspase cascade and mitochondrial dysfunction. Int J Oncol 28(6): 1401–1408.
Kim YS, Seol CH, Jung JW, Oh SJ, Hwang KE, Kim HJ, Kim HR (2015) Synergistic Effect of Sulindac and Simvastatin on Apoptosis in Lung Cancer A549 Cells through AKT-Dependent Downregulation of Survivin. Cancer Res Treat, 47(1):90–100. https://doi.org/10.4143/crt.2013.194
Ko CJ, Lan SW, Lu YC, Cheng TS, Lai PF, Tsai CH, Lee MS (2017) Inhibition of cyclooxygenase-2-mediated matriptase activation contributes to the suppression of prostate cancer cell motility and metastasis. Oncogene, 36(32), 4597–4609. https://doi.org/10.1038/onc.2017.82
Konjalwar S, Ceyhan B, Rivera O, Nategh P, Neghabi M, Pavlovic M, Ranji M (2024) Demonstrating drug treatment efficacies by monitoring superoxide dynamics in human lung cancer cells with time-lapse fluorescence microscopy. J Biophotonics, 17(2): e202300331. https://doi.org/10.1002/jbio.202300331
Li M, Li M, Wei Y, Xu H (2020) Prognostic and clinical significance of cyclooxygenase-2 overexpression in endometrial cancer: a meta-analysis. Front Oncol 10:1202. https://doi.org/10.3389/fonc.2020.01202
Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, Piazza GA (2013) Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/β-catenin signaling. Mol Cancer Ther, 12(9): 1848–1859. https://doi.org/10.1158/1535-7163.Mct-13-0048
Lyndin M, Kravtsova O, Sikora K, Lyndina Y, Kuzenko Y, Awuah WA, Romaniuk A (2022) COX2 Effects on endometrial carcinomas progression. Pathol Res Pract, 238:154082. https://doi.org/10.1016/j.prp.2022.154082
Madka V, Patlolla JMR, Venkatachalam K, Zhang Y, Pathuri G, Stratton N, Rao CV (2023) Chemoprevention of Colon Cancer by DFMO, Sulindac, and NO-Sulindac Administered Individually or in Combinations in F344 Rats. Cancers (Basel), 15(15). https://doi.org/10.3390/cancers15154001
Meyerhardt JA, Shi Q, Fuchs CS, Meyer J, Niedzwiecki D, Zemla T, Shields AF (2021) Effect of celecoxib vs placebo added to standard adjuvant therapy on disease-free survival among patients with stage III colon cancer: the CALGB/SWOG 80702 (Alliance) Randomized Clinical Trial. Jama 325(13), 1277–1286. https://doi.org/10.1001/jama.2021.2454
Miliński M, Staś M, Rok J, Beberok A, Wrześniok D (2023) The effect of sulindac on redox homeostasis and apoptosis-related proteins in melanotic and amelanotic cells. Pharmacol Rep 75(4):995–1004. https://doi.org/10.1007/s43440-023-00493-1
Minami T, Adachi M, Kawamura R, Zhang Y, Shinomura Y, Imai K (2005) Sulindac enhances the proteasome inhibitor bortezomib-mediated oxidative stress and anticancer activity. Clin Cancer Res 11(14):5248–5256. https://doi.org/10.1158/1078-0432.Ccr-05-0085
Mohammed A, Yarla NS, Madka V, Rao CV (2018) Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. Int J Mol Sci, 19(8). https://doi.org/10.3390/ijms19082332
Moon EY, Lerner A (2002) Benzylamide sulindac analogues induce changes in cell shape, loss of microtubules and G(2)-M arrest in a chronic lymphocytic leukemia (CLL) cell line and apoptosis in primary CLL cells. Cancer Res 62(20):5711–5719
Poursoltani F, Nejati V, Pazhang Y, Rezaie J (2021) Sulindac and vitamin D3 synergically inhibit proliferation of MCF-7 breast cancer cell through AMPK/Akt/β-catenin axis in vitro. Cell Biochem Funct 39(8):991–997. https://doi.org/10.1002/cbf.3668
Qu W, Zhao Y, Wang X, Qi Y, Zhou C, Hua Y, Jiang SW (2019) Culture characters, genetic background, estrogen/progesterone receptor expression, and tumorigenic activities of frequently used sixteen endometrial cancer cell lines. Clin Chim Acta, 489:225–232. https://doi.org/10.1016/j.cca.2018.08.013
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48. https://doi.org/10.3322/caac.21763
Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M (2010) The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 2010:215158. https://doi.org/10.1155/2010/215158
Stein U, Arlt F, Smith J, Sack U, Herrmann P, Walther W, Schlag PM (2011) Intervening in β-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia, 13(2): 131–144. https://doi.org/10.1593/neo.101172
Sui HH, Zhou YJ, Wang H, Li L, Cao M, Huang JJ (2018) Effects of sulindac sulfide on proliferation and apoptosis of human breast cancer cell. Oncol Lett 15(5):7981–7986. https://doi.org/10.3892/ol.2018.8331
Sun Y, Tang XM, Half E, Kuo MT, Sinicrope FA (2002) Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res 62(21):6323–6328
Suri A, Sheng X, Schuler KM, Zhong Y, Han X, Jones HM, Bae-Jump VL (2016) The effect of celecoxib on tumor growth in ovarian cancer cells and a genetically engineered mouse model of serous ovarian cancer. Oncotarget, 7(26):39582–39594. https://doi.org/10.18632/oncotarget.8659
Takiuchi T, Blake EA, Matsuo K, Sood AK, Brasky TM (2018) Aspirin use and endometrial cancer risk and survival. Gynecol Oncol 148(1):222–232. https://doi.org/10.1016/j.ygyno.2017.10.026
Thrastardottir TO, Copeland VJ, Constantinou C (2023) The association between nutrition, obesity, inflammation, and endometrial cancer: a scoping review. Curr Nutr Rep 12(1):98–121. https://doi.org/10.1007/s13668-022-00447-8
Toyoshima T, Kamijo R, Takizawa K, Sumitani K, Ito D, Nagumo M (2002) Inhibitor of cyclooxygenase-2 induces cell-cycle arrest in the epithelial cancer cell line via up-regulation of cyclin dependent kinase inhibitor p21. Br J Cancer 86(7):1150–1156. https://doi.org/10.1038/sj.bjc.6600183
Vaish V, Rana C, Piplani H, Vaiphei K, Sanyal SN (2014) Sulindac and Celecoxib regulate cell cycle progression by p53/p21 up regulation to induce apoptosis during initial stages of experimental colorectal cancer. Cell Biochem Biophys 68(2):301–319. https://doi.org/10.1007/s12013-013-9711-8
Vaish V, Sanyal SN (2012) Role of Sulindac and Celecoxib in the regulation of angiogenesis during the early neoplasm of colon: exploring PI3-K/PTEN/Akt pathway to the canonical Wnt/β-catenin signaling. Biomed Pharmacother 66(5):354–367. https://doi.org/10.1016/j.biopha.2012.01.004
Wang YS, Huang NK, Lin YC, Chang WC, Huang WC (2022) Aspirin and Sulindac act via different mechanisms to inhibit store-operated calcium channel: Implications for colorectal cancer metastasis. Biomed Pharmacother 145:112476. https://doi.org/10.1016/j.biopha.2021.112476
Ye Y, Wang X, Jeschke U, von Schönfeldt V (2020) COX-2-PGE(2)-EPs in gynecological cancers. Arch Gynecol Obstet 301(6):1365–1375. https://doi.org/10.1007/s00404-020-05559-6
Zhao H, Yi B, Liang Z, Phillips C, Lin HY, Riker AI, Xi Y (2021) Cyclin G2, a novel target of sulindac to inhibit cell cycle progression in colorectal cancer. Genes Dis 8(3):320–330. https://doi.org/10.1016/j.gendis.2020.11.006
Funding
This research was funded by VLB: NIH/NCI—R37CA226969.
Author information
Authors and Affiliations
Contributions
SC, WK, XS, BD, JH, WS, NS and CJ performed experiments. SC, WK, and CZ analyzed and interpreted the data. SC and CZ prepared the manuscript. CZ and VB designed the experiments, revised the manuscript, and provided financial support. All authors have read and approved the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing potential conflicts of interest for this manuscript. The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, S., Kong, W., Shen, X. et al. Sulindac exhibits anti-proliferative and anti-invasive effects in uterine serous carcinoma cells. J Cancer Res Clin Oncol 150, 402 (2024). https://doi.org/10.1007/s00432-024-05926-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05926-9