Abstract
Purpose
Resistance to chemotherapy and radiotherapy is the primary cause of a poor prognosis in oncological patients. Researchers identified many possible mechanisms involved in gaining a therapy-resistant phenotype by cancer cells, including alterations in intracellular drug accumulation, detoxification, and enhanced DNA damage repair. All these features are characteristic of stem cells, making them the major culprit of chemoresistance. This paper reviews the most recent evidence regarding the association between the stemness phenotype and chemoresistance in head and neck cancers. It also investigates the impact of pharmacologically targeting cancer stem cell populations in this subset of malignancies.
Methods
This narrative review was prepared based on the search of the PubMed database for relevant papers.
Results
Head and neck cancer cells belonging to the stem cell population are distinguished by the high expression of certain surface proteins (e.g., CD10, CD44, CD133), pluripotency-related transcription factors (SOX2, OCT4, NANOG), and increased activity of aldehyde dehydrogenase (ALDH). Chemotherapy itself increases the percentage of stem-like cells. Importantly, the intratumor heterogeneity of stem cell subpopulations reflects cell plasticity which has great importance for chemoresistance induction.
Conclusions
Evidence points to the advantage of combining classical chemotherapeutics with stemness modulators thanks to the joint targeting of the bulk of proliferating tumor cells and chemoresistant cancer stem cells, which could cause recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinomas (HNSCC) are the most common type of neoplastic lesions that develop in the head and neck region. With over 900,000 new cases and over 450,000 deaths in 2020, HNSCC is the eighth most common cancer worldwide (Sung et al. 2021). The incidence of HNSCC continues to rise and is expected to increase by 30% by 2030 (Ferlay et al. 2019). Many patients are diagnosed at an advanced stage of the disease. Thus, they often do not have good long-term prognosis. Like other cancer types, head and neck cancers are managed by surgery, radiotherapy, and chemotherapy (Atashi et al. 2021). Due to the localization of lesions, surgical resection often causes permanent disfigurement and a decrease in quality of life. As a result, survivors of this cancer have the second highest rate of suicide when compared to survivors of other cancers (Osazuwa-Peters et al. 2018). The current standard of care for patients with locally advanced HNSCC is concomitant platinum-based chemoradiotherapy (CRT) or surgery followed by adjuvant radiation or chemoradiation. For patients with recurrent and/or metastatic HNSCC, platinum-based chemotherapy plus 5-fluorouracil (5-FU) has a response rate of 30–40% and a median survival of 6–9 months. Patients with platinum-resistant disease have few options and a very poor prognosis with second-line therapies (Sola et al. 2019). Thus, novel therapeutic strategies augmenting the effects of treatment could significantly benefit HNSCC patients. Furthermore, due to the frequent resistance to conventional treatment, extensive research has been conducted to develop molecularly targeted therapies. So far, only cetuximab, an epidermal growth factor receptor (EGFR) inhibitor, and, more recently, nivolumab and pembrolizumab, PD-1 inhibitors, have been approved for the treatment of HNSCC. However, cetuximab, a monoclonal antibody approved by the FDA in 2006, shows only limited efficacy in advanced HNSCC patients (Sola et al. 2019).
Many factors that contribute to resistance to therapy in HNSCC can be identified. The most studied mechanisms involve alterations in intracellular drug accumulation, detoxification, and DNA damage repair in cancer cells. Other novel mechanisms include epigenetic changes that regulate cell plasticity, the involvement of the tumor microenvironment (TME), and the presence of so-called cancer stem cells (CSCs) (Griso et al. 2022). CSCs constitute a small cell population characterized by slow proliferation, self-renewal capacity by symmetric or asymmetric division, and resistance to therapy (Atashzar et al. 2020; Yang et al. 2020). It is believed that CSCs may be derived from transformed adult stem cells, or they can originate by the dedifferentiation of somatic cells (Barbato et al. 2019; Walcher et al. 2020; Yin et al. 2021). A recent hypothesis states that adult stem cells are the cell population that is most likely to accumulate oncogenic mutations and serve as cancer cells of origin (White and Lowry 2015). The biological behavior of CSCs is determined by the action of several pluripotency and self-renewal-mediating transcription factors, including c-MYC, Nanog, OCT-3/4, SOX2, KLF4, and by the activity of stemness-related signaling pathways, typically Wnt/β-catenin, Hedgehog, Notch, JAK/STAT, TGF-β/SMAD, PI3K/Akt, and NF-kappaB, together with intercellular and extracellular matrix (ECM) communication within the TME niche (Huang et al. 2020a, b; Yang et al. 2020).
CSCs tend to be radio- and chemoresistant, which results from several mechanisms: (1) the upregulated expression of the ABC family of transporters, which are responsible for the exclusion of cytotoxic drugs from cancer cells; (2) the induction of quiescence/dormancy; (3) the enhancement of DNA repair mechanisms; (4) increased protection against oxidative stress; (5) cell plasticity (Barbato et al. 2019; Gupta et al. 2021; Kuşoğlu and Biray Avcı 2019; Yang et al. 2020; Yin et al. 2021). While traditional chemotherapy kills the rapidly dividing cells that constitute the majority of the tumor mass, CSCs may remain intact and cause cancer relapse after the end of treatment (Atashzar et al. 2020; Barbato et al. 2019; Kuşoğlu and Biray Avcı 2019; Walcher et al. 2020). Indeed, chemoresistant cells or cancer stem cells form slow-growing, aggressive/metastatic tumors in mice (Mir et al. 2021). Eliminating CSCs to improve therapy response is not a novel idea, and the scientific community has been exploring these options for decades (Atashzar et al. 2020; Barbato et al. 2019; Gupta et al. 2021; Walcher et al. 2020; Yang et al. 2020). However, this appears to be challenging (Griso et al. 2022).
CSCs in solid tumors are identified by the presence of various cell surface (CD10, CD24, CD44, CD90, CD133, CD271, EpCAM, LGR5) or intracellular (ALDH1, Nanog, OCT3/4, BMI-1, SOX2) markers (Kuşoğlu and Biray Avcı 2019; Walcher et al. 2020). HNSCC cells belonging to the CSC population are distinguished by their high expression of certain surface proteins (for example, CD44, CD133), pluripotency-related transcription factors (SOX2, OCT4, Nanog), and increased aldehyde dehydrogenase (ALDH) activity (Cirillo et al. 2021; Krishnamurthy et al. 2010; Prince et al. 2007). The major markers of HNSCC stem cells are presented in Table 1. Prince et al. (2007) were the first to report the existence of a population of neoplastic cells with stem cell properties in HNSCC (Prince et al. 2007). They observed that the CD44 + cell population could form new tumors in vivo, in contrast to the CD44 – cell population. Moreover, CD44 + cells were less differentiated than CD44 – cells, which more closely resembled a differentiated squamous epithelium and showed increased involucrin expression (a marker of keratinocyte differentiation). Tumors that arose from the isolated population of CD44 + cells recreated the heterogeneity of the primary tumor and could be passaged multiple times, which proved that the CD44 + population had two key stem cell features—the ability for differentiation and self-renewal. Krishnamurthy et al. (2010) showed that, based on the assessment of ALDH activity within the CD44 + tumor cell population, it is possible to identify a subpopulation of cells with an even greater intensity of features typical of stem cells, which constituted 1–3% of the cells of the primary tumor (Krishnamurthy et al. 2010). In their studies, the implantation of 1000 CD44 + /ALDH + cells led to the formation of tumors in 13 out of 15 mice, while the implantation of 10,000 CD44 –/ALDH – cells led to the development of tumors in only two animals. ALDH1 activity has also been associated with an increased frequency of local relapse after the end of therapy (Ota et al. 2014). Later studies have shown that these stem-like cells are also much less sensitive to chemoradiotherapy and can persist after therapy, leading to relapse. The overexpression of CD44 correlated with poor overall and disease-free survival in patients with advanced oral carcinomas (Boxberg et al. 2018). In addition, the expression of ALDH1 and CD44 was a predictor of angiolymphatic invasion and lymph node metastasis in patients with oral carcinomas, respectively (Ortiz et al. 2018). In another study, ALDH1 expression was associated with lymph node involvement and high mortality rate (Gupta et al. 2022). A broader stem cell gene expression signature correlated with lower 5-years and relapse-free survival rates in HPV-negative HNSCC patients (Kim et al. 2022). HNSCC tumors developing in Fanconi anemia patients carry a very poor prognosis and require aggressive treatment. Notably, these tumors contain a greater proportion of ALDH-positive CSC cells showing Nanog and Oct-3/4 expression, in comparison with sporadic HNSCC (Wu et al. 2014). Thus, current evidence shows that an increase in cancer stem cell population confers poor prognosis in HNSCC.
The growing field of research on the importance of cancer stem cells in HNSCC has resulted in many new and interesting findings in recent years. This narrative review aimed to present the latest evidence documenting the significance of cancer stem cells in the development of therapeutic resistance in head and neck squamous cell cancers, including the molecular mechanisms involved in the stemness-related development of resistance. We searched the PubMed database using the keywords “head neck cancer stem cell resistance” and retrieved records from the last decade (Jan 2012–Feb 2023). We identified additional records by cross-references. Our analysis included all the experimental papers that tested the association between stemness potential and resistance. Most studies have focused on the chemoresistance to conventional chemotherapeutics, mainly cisplatin, but also 5-fluorouracil or docetaxel. We excluded papers that merely reported an association between resistance phenotype and the expression of stemness markers, and we focused on papers that reported evidence generated with the use of stem cell subpopulations. Aiming to focus on squamous cell carcinomas, we also excluded papers studying esophageal, thyroid, salivary gland, nasopharyngeal or central nervous system tumors, because of different etiological and clinical factors related to these tumor types. Additionally, we excluded experimental papers that used the misidentified Hep2 cell line as a model of HNSCC.
Several review papers have recently been published on this topic (Cirillo et al. 2021; Mudra et al. 2021; Siqueira et al. 2023), but new information has appeared since. This review presents up-to-date knowledge and focuses on the possibilities of pharmacological targeting of stemness-related chemoresistance. More information about the relationship between stemness and radioresistance can be found in other excellent reviews (Atashzar et al. 2020; Siqueira et al. 2023).
The association between stemness phenotype and chemoresistance in HNSCC
Chemotherapy leads to the enrichment of cancer stem cells
Several lines of evidence point to the appearance of cancer stem cells as the driving force in chemoresistance. First of all, many studies have shown that chemotherapy and radiotherapy can increase the percentage of cancer stem cells. In this regard, cisplatin led to an increase in the percentage of ALDH-positive cells (Kim et al. 2017; Subramanian et al. 2017) or CD44high cells (Basak et al. 2015; Bu et al. 2015), or ALDHhighCD44high cells (Nakano et al. 2021; Nör et al. 2014). Cells identified as the side population (SP) exhibit the ability to efflux the Hoechst33342 dye, which is a measure of drug efflux capability reflecting cellular chemoresistance. To a large extent, these cell populations overlap with stem-like cells (Yang et al. 2020). Cisplatin has been shown to increase the ratio of CD44 + cells or the percentage of SP cells, which show elevated expression of CD44, CD133, ALDH1A, and ABCG2 (Jiang et al. 2018). In addition, cisplatin increased the SP and CD24 + cell populations (Sinnung et al. 2021). Moreover, ionizing radiation increases the percentage of side population cells (Macha et al. 2017). Thus, in general, chemoradiotherapy leads to a dangerous enrichment with CSCs, which poses a significant risk of treatment failure in the long term (Dzobo et al. 2020). It remains unclear whether this is solely a consequence of killing the bulk of proliferating cancer cells or whether chemotherapeutics can transform cells toward a stem-like phenotype. Although this is difficult to discern experimentally, some evidence points to the possibility of the latter (Nör et al. 2014; Vipparthi et al. 2022). Moreover, stem cell plasticity may be responsible for the adaptive response to chemotherapy, leading to resistance (Gupta et al. 2021). HNSCC cell lines and tumor-derived cells exhibit different stem cell subpopulations based on the presence of CD44, CD24, and ALDH markers. CD44high cells may transition into CD44highALDHhigh cells or CD44highCD24high cells, and the latter could also gain ALDH activity. It has been observed that while the CD24 transition was unidirectional, there was plasticity/reversibility on the ALDH axis. Notably, the acquisition of cisplatin resistance was related to stem cell phenotype switching. Cisplatin induced the transition toward CD24high cells and stimulated plasticity toward the ALDHhigh subpopulation. Indeed, triple-positive cells (CD44highCD24highALDHhigh) were the most enriched subpopulation after cisplatin treatment, presenting a highly cisplatin-tolerant phenotype associated with high expression of ABCG2 drug efflux protein (Vipparthi et al. 2022).
Many studies focused on cancer stem cells used the tumorsphere assay to evaluate the effects on chemosensitivity. This assay is a simple measure of stemness-associated self-renewal under low-attachment conditions and is utilized to enrich the subpopulation of cancer stem cells (Yang et al. 2020). Cisplatin was shown to increase the efficiency of sphere formation in the tumorsphere assay (Nör et al. 2014; Subramanian et al. 2017). Indeed, cells grown as spheres showed an increased level of CD44, SOX2, OCT4, NANOG, and c-Myc, compared to monolayer cells (Huang et al. 2020a, b). SAS cells grown as spheres were less sensitive to cisplatin or gemcitabine than parental cells (Sun et al. 2022a, b). Moreover, stem cells isolated from SAS cells orospheres were much less sensitive to cisplatin than parental cells (Peng et al. 2022). Additionally, HNSCC stem cells generated by growing parental cells as spheres for three generations showed elevated expression of ALDH1, SOX2, and KLF4 and lowered sensitivity to cisplatin or 5-fluorouracil (Garcia-Mayea et al. 2019). Thus, there is a clear association between the exposure to cytostatic drugs and cancer stem cells accummulation, either because of their selection following the killing of bulk cancer cells, or due to stimulation of cell plasticity, or both.
Drug-resistant cells show the enrichment in stem cell subpopulations
Another line of evidence comes from studies using resistant cell lines generated in vitro by prolonged sequential treatment of cells with increasing drug concentrations. Such resistant cells exhibit enhanced stemness compared to the parental cells. For example, cisplatin-resistant CAL27 and SCC-131 cells were able to form larger tumorspheres, pointing to higher self-renewal potential, and exhibited elevated expression of CD44, KLF4, OCT4, SOX2, c-MYC, and β-catenin (Roy et al. 2018, 2019). Similarly, cisplatin-resistant OC2 cells showed a greater capacity for tumorsphere formation and increased expression of CD133, ABCG2, BMI1, OCT4, and NANOG (Tsai et al. 2011). Multidrug (cisplatin, docetaxel, doxorubicin, erlotinib) resistant HSC-3 cells showed higher expression of CD44 and SOX9 and increased ability for tumorsphere formation (Murakami et al. 2022). Furthermore, CAL27 cells resistant to cisplatin or docetaxel were enriched in CD44 + cells and showed elevated expression of CD133, ALDH1A1, OCT4, SOX2 (Kulsum et al. 2017), and cisplatin-resistant CAL27, and SCC9 cells showed the accumulation of CD44 + ALDH + cells (Lima de Oliveira et al. 2022). In addition, cisplatin-resistant FaDu cells showed increased expression of CD44 and an increased percentage of CD44-positive cells. They also exhibited increased autophagy, which inhibition with anti-ATG14 siRNA reduced CD44 expression (Naik et al. 2018). On the other hand, cisplatin-resistant Detroit 562 cells are enriched in CD10-positive cells (Fukusumi et al. 2014). Cisplatin-resistant SAS cells showed higher ALDH activity and increased expression of CD133, OCT4, and NANOG (C.-W. Chang et al. 2014). Similarly, cisplatin-resistant UM-SCC-22B cells exhibited higher expression of BMI1 and OCT4 pluripotency markers (Nör et al. 2014). Cisplatin-resistant SCC-4/-9 cells showed elevated expression of NANOG, which transcriptionally stimulated OCT4, c-MYC, and ABCG2 expression, which was reduced by NANOG knockdown, leading to sensitization to cisplatin (Kashyap et al. 2020). Moreover, immunohistochemical analysis showed the upregulation of OCT4 and NANOG in OSCC patients characterized by chemoresistance, which indicates that these in vitro findings have clinical relevance (Tsai et al. 2011). Thus, it can be concluded that chemoresistant cells are characterized by the accumulation of cancer stem cells and the increased expression of stemness (CD44, CD133, CD10, ALDH) and pluripotency (NANOG, BMI1, OCT4, SOX2) markers. Thus, the acquisition of cellular chemoresistance is indeed associated with increased stemness potential.
Isolated cancer stem cells are resistant to chemotherapeutics
The most compelling evidence for the key importance of targeting cancer stem cells in tackling chemoresistance comes from studies that used the subpopulations of CSCs isolated by selective cell sorting based on the presence of stem cell markers. Since no single marker of HNSCC stem cells exists, studies focused on analyzing different stemness-related proteins, with CD44 and ALDH being the most frequently investigated. For example, ALDH + cells showed higher expression of BMI1, OCT4, NANOG, and MDR1 and reduced radiosensitivity (Chen et al. 2010). In another study, a small subpopulation of UTSCC-60A cells that were positive for ALDH expressed higher levels of BMI1, KLF4, OCT4, and SOX2 and were resistant to cisplatin (Gunduz et al. 2019). In parallel, ALDHlow cells were more sensitive to paclitaxel (Fernandes et al. 2022). ALDH-positive cells are usually described as a subpopulation of CD44high cells, and these cells were resistant to docetaxel or cetuximab (Keysar et al. 2017). Moreover, a subpopulation of CD44v3highALDHhigh HNSCC stem cells, which expressed OCT4, NANOG, and SOX2, was resistant to apoptosis induction because of the high expression of IAP proteins (XIAP, c-IAP2). The presence of hyaluronic acid (HA), which interacts with CD44, further decreases cisplatin-induced apoptosis, whereas anti-CD44 antibody sensitizes cells to cisplatin (Bourguignon et al. 2012, 2016). This underscores the role of the HA-CD44 axis in HNSCC chemoresistance. Histopathological analyses seem to corroborate these findings because the increased immunohistochemical levels of ALDH1, CD44, or pSTAT3 were associated with shorter overall survival in HNSCC patients, while the worst survival rate was observed in triple-positive patients (Chen et al. 2010). Additionally, CD44high cells showed lower proliferation but higher colony formation ability and were resistant to cisplatin, and tended to be resistant to EGFR inhibition by cetuximab or gefitinib (La Fleur et al. 2012). CD44 + HNSCC cells were resistant to apoptosis induction, and showed elevated expression of anti-apoptotic Bcl-2 and IAP proteins (Chikamatsu et al. 2012). The ratio of CD44 + cells significantly varies among different HNSCC cell lines and not all CD44 + cells exhibit stem-like properties and chemoresistance (Modur et al. 2016). Furthermore, subpopulations of CD44high cells were distinguished based on differences in cell morphology, and ameboid-like CD44high cells showed significant resistance to docetaxel, compared to epithelial-like or mesenchymal-like CD44high OSCC cells (Yokoyama et al. 2021). In another study, mesenchymal-like CD44high cells, which appeared when cells were grown on fibronectin-coated hydrogel, were characterized by elevated expression of NANOG, SOX2, OCT4, and ALDH1 and resistance to cisplatin when compared to epithelial-like CD44high or parental cells (Shigeishi et al. 2022). Thus, it is relevant to recognize that CD44high cells are neither homogeneous nor a fixed population of cells. Indeed, stemness seems to depend on cell plasticity and constitutes the feature of the tumor as a whole (Wang et al. 2017). On the other hand, in vitro growth conditions affect stem cells by inducing adaptive changes due to cell plasticity. It remains to be established which culture conditions are best in mimicking the in vivo environments to allow the best possible prediction of therapeutic response.
CD271 belongs to the TNF family of receptors, and it is present in the stem cells of the normal oral epithelium. However, its growing expression has been observed in various stages of pathology: from dysplasia to advanced HNSCC cases (Elkashty et al. 2020). Interestingly, the presence of CD271 is restricted to CD44 + cells and CD44 + CD271 + cells turned out to have the highest tumorigenic potential (Murillo-Sauca et al. 2014). Furthermore, CD44 + CD271 + cells exhibited a higher capacity for tumorsphere formation and increased expression of BMI1, OCT4, and SOX2 and showed resistance to cisplatin and, to a lesser extent, 5-fluorouracil (Elkashty et al. 2020). In an interesting study using patient-derived xenotransplanted hypopharyngeal tumors, cell sorting led to the identification of a subpopulation of tumor-initiating cells that were positive for CD271. These cells would tend to be located in tumors at the invasive front and near blood vessels. They were also highly tumorigenic in mice. Moreover, CD271 + cells expressed NANOG, OCT4, and SOX2 and cell surface efflux transporters, e.g., ABCC2, ABCB5, and ABCG2. Importantly, cisplatin was able to kill CD271-negative cells while CD271-positive cells survived cisplatin treatment in vivo. This suggests that the presence of CD271 marks a subpopulation of cisplatin-resistant HNSCC stem cells (Imai et al. 2013).
Although rarely detected in HNSCC samples (Fukusumi et al. 2014), CD133 is yet another marker of stem cells. A small percentage of HNSCC-derived cells were characterized as SP cells which expressed stemness markers (CD133 and OCT4) and showed high self-renewal capacity. These cells were resistant to cisplatin, oxaliplatin, paclitaxel, and 5-fluorouracil, which was associated with increased expression of ABCG2 drug efflux transporter and anti-apoptotic Bcl2 (Guan et al. 2015; Lu et al. 2016). In another study, CD44highCD133highCD117high HN13 cells were much less sensitive to paclitaxel treatment than parental cells (viability 88% vs 44%, respectively) (Silva Galbiatti-Dias et al. 2018).
Moreover, several studies have shown that HNSCC stem cells also exhibit the presence of CD10 or CD24 surface markers. Indeed, CD10 + cells formed more tumorspheres, expressed higher levels of ALDH1 and OCT3/4, and were tumorigenic in mice. These slow-cycling dormant cells were resistant to cisplatin, 5-fluorouracil, and radiation (Fukusumi et al. 2014). Additionally, CD10high cells showed higher expression of ALDH1, BMI1, OCT4, NANOG, and SOX2 and were significantly less sensitive to cisplatin, than CD10low cells (Pu et al. 2021; Wang et al. 2021). In addition, higher expression of CD24, NANOG, and OCT4 correlated with a reduced response to cisplatin combined with radiotherapy in patients with OSCC (Mishra et al. 2020). Furthermore, the percentage of CD24 + cells correlated with cisplatin resistance in HNSCC cell lines, and CD24 knockdown significantly reduced NANOG expression and sensitized cells to cisplatin treatment. Moreover, CD24 + cells were enriched in the fraction of residual resistant cells (Modur et al. 2016).
The pharmacological targeting of stemness-mediated HNSCC chemoresistance mechanisms
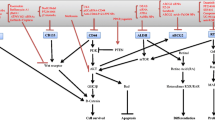
A complex network of molecular pathways regulates the transcriptional and cellular programs responsible for the stemness phenotype. Thus, many potential molecular targets (Fig. 1) could be therapeutically modulated to prevent relapse by facilitating the elimination of chemoresistant cancer stem cells and thus increasing the effectiveness of chemotherapeutics.
The molecular mechanisms responsible for stemness-associated resistance (Bu et al. 2015; Chen et al. 2017, 2022, 2010; Garcia-Mayea et al. 2020; Han et al. 2021; Herzog et al. 2021; Hsueh et al. 2021; Huang et al. 2020a, b; Jang et al. 2022; Jiang et al. 2018; Kashyap et al. 2018; Keysar et al. 2017; Lee et al. 2016; Lim et al. 2014; Paluszczak 2020; Peng et al. 2018; Silva Galbiatti-Dias et al. 2018; Sinnung et al. 2021; Song et al. 2022; Wang et al. 2017, 2018; Zhao et al. 2022). Abbreviations: c-MET tyrosine-protein kinase Met, DOT1L DOT1 like histone lysine methyltransferase, HA hyaluronic acid, HDAC1/2 histone deacetylase 1/2, HGF hepatocyte growth factor, HMGA2 high mobility group AT-hook 2, IL-4,6,8 interleukin-4,6,8, ILRs interleukin receptors, KRT17 keratin 17, LSD1 lysine-specific demethylase 1, NFAT nuclear factor of activated T-cells, PLEK2 pleckstrin 2, PLOD2 procollagen lysine 2, RTKs receptor tyrosine kinases, SRC proto-oncogene tyrosine-protein kinase Src, SDCBP syntenin-1, STAT3 signal transducer and activator of transcription 3, TET1 tet methylcytosine dioxygenase 1, TSPAN1 tetraspanin-1, TRPM7 transient receptor potential cation channel, subfamily M, member 7
PI3K/Akt, Wnt/β-catenin and Src pathways interactively induce stemness-related resistance
ALDH + CD44high cells showed activation of the PI3K/Akt/mTORC1 pathway, which regulates SOX2 expression, in turn activating ALDH1A1 expression and activity. These cells responded to PI3K inhibition, which decreased the ALDH + population and SOX2 expression without affecting CD44 expression. Moreover, SOX2 overexpression diminished the response to docetaxel (Keysar et al. 2017). The Akt kinase activation can also be mediated by PDK1, whose knockdown reduced the level of pAkt, and affected stemness by reducing the expression of SOX2, OCT4, and CD133, decreasing tumorsphere formation capacity. Moreover, the PDK1 inhibitor—BX795—sensitized OSCC cells to cisplatin (Pai et al. 2021). Thus, the PI3K/Akt pathway is mechanistically responsible for the induction of ALDH and SOX2 expression and participates in stemness-related chemoresistance. These effects may be mediated by cross-talk with other signaling pathways. The canonical Wnt/β-catenin pathway has been implicated in HNSCC development (Paluszczak 2020), and a recent study has shown that pAkt stimulates β-catenin nuclear translocation, which induces the TCF4-mediated transcription of ALDH1A1 (Wang et al. 2017). Moreover, the activation of the Wnt/β-catenin pathway plays a role in the cisplatin-induced enrichment of resistant stem cells (Sinnung et al. 2021). Cisplatin-resistant and CSC cells showed the elevated level of β-catenin and EZH2. EZH2, which is a histone methyltransferase mediating transcriptional repression by H3K27 methylation, suppressed APC which acts as the upstream inhibitor of β-catenin activation. The combinatorial inhibition of both Wnt/β-catenin and EZH2 effectively reduced the CSC population in vitro and in vivo, and sensitized cells to cisplatin (Milan et al. 2023). Notably, the Wnt pathway also influences RXR-mediated effects. In this regard, the overexpression of RXRα or the addition of retinoids (9-cis-retinoic acid) led to the enrichment of SP cells and CD44 + cells, and increased the level of expression of stemness markers (CD44, CD133, SOX2, OCT4), while the knockdown of RXRα resulted in the opposite effects (Jiang et al. 2018).
On the other hand, a pathway initiated by the interaction of Keratin 17 (KRT17) with plectin and integrin-64 may stimulate the transcriptional activity of β-catenin. This pathway activates the FAK/Src/ERK cascade of downstream kinases, ultimately resulting in the nuclear translocation of β-catenin, leading to the upregulation of CD44 and enhanced sphere formation. Importantly, the knockdown of KRT17 reduced the self-renewal potential and sensitized cells to cisplatin (Jang et al. 2022). In addition, cisplatin-resistant cells expressed higher levels of tetraspanin-1 (TSPAN1), and its siRNA-mediated reduction enhanced susceptibility to cisplatin and dasatinib. Dasatinib is a small molecule inhibitor targeting the Src pathway. Indeed, TSPAN1 depletion reduced the level of active phospho-Src kinase, although TSPAN1 targeted both Src-dependent and independent pathways (Garcia-Mayea et al. 2020). Similarly, syntenin-1 (SDCBP) was upregulated in cisplatin-resistant and stem-like Detroit 562 cells, and the depletion of SDCBP sensitized the cells to cisplatin. This led to reduced expression of CD44, CD133, KLF4, and OCT3/4 and decreased levels of phospho-Src protein. Moreover, Src inhibition also sensitized cells to cisplatin (Mir et al. 2021). These data would suggest that Src may be an important downstream effector regulating stemness and chemoresistance in HNSCC. Indeed, the inhibitors of the Src family of kinases are an emerging group of anti-cancer molecularly targeted therapeutics. However, they did not demonstrate sufficient clinical effectiveness in HNSCC. In contrast to the aforementioned studies, a recent paper has shown that Src inhibitors—dasatinib or saracatinib—not only failed to eliminate cancer stem cells in tumorspheres but also increased the expression of ALDH1A1, SOX2, OCT4, and NANOG. The authors of this work hypothesized that this pro-stemness activity was responsible for the poor clinical response to these drugs. A mithramycin analog, EC-8042, on the other hand, reduced the stemness phenotype, and the combination of this compound with dasatinib was beneficial. Such simultaneous targeting of proliferating and migrating cells by dasatinib and tumor-propagating cells by EC-8042 led to potent antitumor activity in vivo (Hermida-Prado et al. 2019). Thus, the exact role of Src signaling in regulating stemness and pluripotency in HNSCC cells still requires elucidation.
The activation of the MEK/ERK pathway has been shown to contribute to cisplatin resistance by inducing the expression of CD44v4 and its pharmacological inhibition reversed this phenotype (Kashyap et al. 2018). In another study, the ERK1/2 pathway induced the expression of CD44 and NANOG and increased resistance to cisplatin or 5-fluorouracil in CD44 + cell spheroids. ERK inhibitors sensitized these cells to chemotherapeutics (Huang et al. 2020a, b). On the other hand, MEK/ERK inhibition did not affect ALDH expression (Keysar et al. 2017). The inhibition of p38 using SB203580 reduced the RNA and protein levels of stemness markers (CD44, OCT4, KLF4) in cisplatin-resistant SCC-131 and CAL27 cells. In addition, pretreatment of cells with the p38 inhibitor sensitized resistant cells to cisplatin, significantly increasing DNA damage and apoptosis. Moreover, SB203580 prevents cisplatin-induced enhancement of stemness marker expression (Roy et al. 2021).
Interleukins induce stemness-related resistance via STAT3 activation
Several interleukins (IL) have been implicated in regulating stemness and resistance. Indeed, cisplatin-resistant HNSCC cells showed higher expression of IL-6/8/10 (Basak et al. 2015). Also, IL6 enhanced cisplatin-induced enrichment of the ALDHhighCD44high cells (Nör et al. 2014). The low level of let-7c in ALDH + and CD44 + cells was responsible for the upregulation of IL-8 secretion. Conversely, the overexpression of let-7c attenuated IL-8 level, reduced ALDH activity and sensitized ALDH + cells to cisplatin. However, the addition of IL-8 could antagonize these effects (Peng et al. 2018). Moreover, the increased secretion of IL-8 via ERK signaling activation enhanced chemoresistance in cisplatin-resistant CD10high cells. IL-8 inhibition using SB225002 sensitized CD10high cells to cisplatin (Pu et al. 2021). Additionally, it has been found that the Hedgehog signaling pathway is involved in regulating the cisplatin-resistant properties of CD10high cells (Y. Wang et al. 2021). Also, the hypersecretion of IL-4 can drive the multidrug resistance phenotype of CD133 + side population cells and neutralizing IL-4 by antibody sensitized these cells to drug treatment (Guan et al. 2015). The cross-talk between JMJD6 and IL-4 further substantiates the importance of IL-4 for stemness. It has been shown that tumorsphere cells express higher levels of several histone demethylases, including JMJD6. Indeed, ALDHhigh cells showed elevated expression of JMJD6. On the other hand, JMJD6 overexpression led to increased expression of stemness markers (OCT4, NANOG) and resulted in cell resistance to doxorubicin, etoposide, and methotrexate. Importantly, JMJD6 transcriptionally regulates IL-4. Anti-IL-4 antibodies suppressed the stem-like phenotype of JMJD6 overexpressing cells, while recombinant IL-4 rescued the stemness phenotype (C.-R. Lee et al. 2016). The association between immunomodulation, stemness, and chemoresistance is further supported by the observation that the upregulation of the CXCR3A chemokine receptor increased the expression of SOX2 and NANOG, and stimulated the resistance to cisplatin, gemcitabine, and paclitaxel (Sun et al. 2022a, b).
The activation of the STAT3 pathway, which can be mediated by IL-4, IL-6, or other factors, was shown to maintain the stemness potential of radioresistant ALDH + CD44 + cells. The decrease in phospho-STAT3 levels induced by cucurbitacin I stimulates the differentiation of these stem cells into ALDH/CD44-negative cells, sensitizing tumors to ionizing radiation (Chen et al. 2010). The activation of STAT3 correlated with the increased expression of ALDH1, CD44, OCT4, and SOX2 in cancer cells (Bu et al. 2015). Moreover, it has been shown that the activation of the IL-6/STAT3 pathway in cisplatin-resistant cell lines is driven by collagen lysyl hydroxylase PLOD2, which results in the stimulation of the expression of stemness markers CD44 and CD133 via integrin β1 (Song et al. 2022). PLOD2 can also activate Wnt signaling and PLOD2 overexpressing FaDu cells showed elevated expression of NANOG, OCT4, and KLF4. PLOD2 knockdown reduced the tumorsphere forming capacity, the percentage of SP cells, and sensitized cells to cisplatin treatment (Sheng et al. 2019). The silencing of IL-6R decreased the percentage of cisplatin-induced ALDHhighCD44high cells showing that IL-6/STAT3 signaling is important for the stemness phenotype by regulating the expression of BMI1 (Herzog et al. 2021). Magnololol-induced sensitization of orosphere-derived stem cells from the SAS cell line to cisplatin is mediated by the reduced secretion of IL-6 and decreased activation of STAT3. Moreover, magnolol attenuated ALDH activity and decreased the capacity for secondary sphere formation (Peng et al. 2022). The inhibition of STAT3 using S3I-201 led to the elimination of both bulk and side population cancer cells in vitro. Also, it diminished the capacity for tumorsphere formation and the expression of ALDH1, CD44, OCT4, and NANOG, resulting in the sensitization of cells to chemotherapeutics (Bu et al. 2015).
Other players
TRPM7 is a membrane protein that functions as a channel for divalent cations (particularly Mg2 +) and contains a serine/threonine kinase domain. The protein acts as a sensor of changes in cellular osmolarity, and pH alterations. It has pleiotropic functions, and affects cell survival, proliferation and migration. It has been shown that cisplatin-resistant patients show higher RNA expression of TRPM7. The downregulation of this membrane receptor protein was associated with a decrease in the expression of stemness markers (BMI1, OCT4, SOX2, NANOG) in SAS cells. The knockdown of TRPM7 in combination with cisplatin strongly reduced the capacity for tumorsphere formation. These findings suggest that the TRPM7/NFAT pathway is relevant for maintaining OSCC stem cells (Chen et al. 2022).
Pleckstrin-2 (PLEK2) is another protein that is implicated in the regulation of stemness. Pleckstrin-2 is a cell membrane-associated protein which takes part in focal adhesion and contact with the actin cytoskeleton, and is also implicated in PI3K signaling. PLEK2 was found overexpressed in dysplasia and HNSCC, showing the highest expression in chemoresistant patients. Also, chemoresistant cell lines expressed higher levels of PLEK2. The overexpression of PLEK2 increased the proportion of ALDH + cells, while the knockdown of PLEK2 reduced the expression of stemness markers (CD133, BMI1, SOX2, OCT4, NANOG) and decreased ALDH activity. These effects were mediated by the stabilization and activation of c-MYC by PLEK2 (Zhao et al. 2022).
The HGF/c-MET pathway also plays a role in the maintenance of stemness phenotype and chemoresistance. Indeed, ALDHhigh cells showed high expression of c-Met, while c-Methigh cells were characterized by the increased expression of OCT4, SOX2, and CD44. The knockdown of c-Met decreased the expression of these stem cell markers and diminished tumorsphere forming capacity. Moreover, it led to a decrease in the percentage of SP cells and reduced the expression of ABCG2 transporter protein, which was associated with modest cisplatin sensitization (Lim et al. 2014).
BMI1 is one of the pluripotency markers whose enhanced expression seems to play an important role in HNSCC cell stemness. CD44 + ALDHhigh cells isolated from parental and cisplatin-resistant SCC-1 cells showed elevated expression of BMI1. Also, tumor-derived EpCAM + CD44 + ALDHhigh cells showed elevated BMI1 expression. Indeed, BMI1 + cells were found to be slowly proliferating but could transform into actively proliferating cells, which points to their stem-like features. BMI1 + cells isolated from primary tumors showed high clonogenic potential, as shown by the ability to form primary and secondary tumorspheres. Also, they were highly tumorigenic in vivo, in contrast to BMI1 non-expressing cells. BMI1 has been associated with chemoresistance since PTC-209, an inhibitor of BMI1, restored the sensitivity of cisplatin-resistant SCC-1 cells to cisplatin. This points to the possible clinical potential of combining classical chemotherapeutics with stemness modulators thanks to the joint targeting of the bulk of proliferating tumor cells and chemoresistant cancer stem cells. Monotherapy with cisplatin killed mitotic cells and induced apoptosis of BMI-negative cells while enriching BMI + cells that were present in recurrent or persistent tumors in the mouse 4NQO-induced tumor model. This shows that the lack of elimination of cancer stem cells is responsible for treatment failure. Importantly, the combination therapy with cisplatin and PTC-209 effectively inhibited tumor growth. PTC-209 significantly decreased the percentage of BMI + cancer stem cells and its combination with cisplatin reduced both BMI1 + and bulk cancer cells in vivo. Importantly, a similar effect was observed with the AP-1 inhibitor, 3-PA, which underscores the importance of the AP1 pathway in regulating BMI1 expression (Chen et al. 2017).
CAL27 and FaDu cells grown as tumorspheres showed elevated expression levels of CD133, CD44, ALDH1, SOX2, and BMI1. The higher expression of these stemness markers may depend on the activity of the HMGA2 protein, which acts in cooperation with Slug. Knockdown of HMGA2 reduced the expression of CD133, CD44, ALDH1, SOX2, and BMI1, and diminished tumorsphere formation capacity. On the other hand, overexpression of HMGA2 increased tumorsphere formation and facilitated cell survival in the presence of cisplatin, thus causing chemoresistance (Li et al. 2022). Chaperone proteins are another player in therapy resistance. The pharmacological inhibition of Heat shock protein 90 (Hsp90) with KU711 or KU757 decreased the number of spheres and the percentage of ALDH-positive and CD44-positive cells and the level of BMI1 protein in parental and cisplatin-resistant HNSCC cell lines (Subramanian et al. 2017).
Epigenetic regulation of transcription and CSC chemoresistance
Epigenetic mechanisms can affect transcriptional programs associated with cell plasticity and the induction of the expression of pluripotency and stemness-related genes. For example, CD44 may lead to chemoresistance by increasing the expression of anti-apoptotic IAP proteins. These effects are mediated by the upregulation of DOT1L histone lysine methyltransferase and the subsequent increase in the methylation level of H3K79 residue, which directs the activation of gene transcription (Bourguignon et al. 2016). The observation that the knockdown of TET1 protein, which is responsible for active DNA demethylation, may sensitize CD44 + cells to cisplatin further supports the association between stemness-related chemoresistance and epigenetic mechanisms. TET1 promotes chemoresistance by MGMT promoter demethylation, augmenting DNA repair response to damages induced by alkylating agents (Wang et al. 2018). Moreover, cisplatin-resistant cell lines showed the overexpression of histone deacetylases HDAC1/2 (Lima de Oliveira et al. 2022). On the other hand, histone lysine demethylase LSD1 was essential for the stimulation of the expression of BMI1. LSD1 knockdown suppressed stemness characteristics, although it led to the upregulation of PDL1, enhancing immune evasion. However, the combination of LSD1 inhibition and PD-1 blockade showed efficacy in vivo, leading to overcoming immune evasion (Han et al. 2021).
Redox states and CSC chemoresistance
Stemness and resistance are also associated with redox homeostasis. For instance, cisplatin was found to elevate the proportion of stem-like ROSlow cells. Cisplatin-resistant SAS cells exhibited low levels of reactive oxygen species (ROS) due to increased expression and activity of catalase, superoxide dismutase 2 (SOD2), or peroxiredoxin. Thus, the depletion of ROS scavengers may stimulate chemosensitivity. Indeed, cell treatment with 2-metoxyestradiol and/or 3-amino-1,2,4-triazole lowered the expression of OCT4 and NANOG and reduced the proportion of ROSlow cells, thus sensitizing cells to cisplatin (Chang et al. 2014). Interestingly, FaDu cells that acquired resistance to PI3K inhibitor BEZ235 and cross-resistance to gefitinib and cisplatin exhibited stemness phenotype. Specifically, these cells had elevated activity of ALDH and increased expression of NANOG, OCT4, SOX2, and BMI1 but also displayed ROS imbalance and SOD2 upregulation. Notably, SOD inhibitors sensitized these resistant cells to BEZ235 (Hsueh et al. 2021). Also, cisplatin-resistant CAL27 and SCC9 cells demonstrated reduced ROS levels. The inhibition of HDAC6 by tubastatin A induced oxidative stress in these cells, reversing the cisplatin-induced accumulation of CD44highALDHhigh stem cells (Tavares et al. 2022). Moreover, increasing ROS formation by the inhibition of ALDH activity with Aldi-6 contributed to cell sensitization to cisplatin, which could be counteracted by the addition of antioxidant N-acetylcysteine (Kim et al. 2017). Additionally, the chemoresistant CD133 + side population cells exhibited increased expression of the Nrf2 transcription factor, which promotes the expression of cytoprotective and antioxidant proteins (Lu et al. 2016). These findings indicate the significant contribution of redox imbalance in the acquisition and/or maintenance of chemoresistance in stem-like HNSCC cancer cells.
The important role of tumor microenvironment
Solid tumors consist of multiple cell types, and recent evidence points to the crucial role of alterations in TME for epithelial neoplastic transformation (White and Lowry 2015). Apart from the heterogenous clones of neoplastic cells (both bulk and stem-like cells), non-neoplastic cells, including fibroblasts, macrophages, mesenchymal stem cells, endothelial cells and immune cells, are also present in TME (Dzobo 2020; Dzobo et al. 2023; Kok 2020). These stromal cells infiltrate the tumor and become hijacked by cancer cells to support tumor growth and drug resistance, thus pointing to the inhibition of tumor-stroma interactions as a key target in chemosensitization (Senthebane et al. 2017). The interaction between cancer cells and stromal cells is multidirectional, and cancer stem cells continuously interact with these cells to establish a favorable niche (Fig. 2) (Dianat-Moghadam et al. 2023; Huang et al. 2020a, b). Tumor-associated macrophages (TAMs) increased CSC fraction by elevating the level of hyaluronic acid in ECM, and subsequent stimulation of CD44/PI3K pathway (Gomez et al. 2020). The significance of such intercellular cross-talk in drug resistance acquisition may be indirectly confirmed by an observation that stronger infiltration of HNSCC tumors with TAMs predicted worse response to chemoradiotherapy and was associated with higher risk of relapse (Balermpas et al. 2014).
Tumor microenvironment niche. Fibroblasts, macrophages and mesenchymal stem cells infiltrate tumors and are hijacked by cancer cells to support tumor survival and growth by promoting stemness, dormancy, immunoediting, and by altering the structure of the extracellular matrix. All these result in chemoresistance (Dianat-Moghadam et al. 2022, 2023; Dzobo 2020; Dzobo et al. 2023; Gupta et al. 2021; Jingyuan et al. 2023; Kok 2020; Senthebane et al. 2018, 2017)
Most stromal cells are able to secrete pro-tumorigenic factors, including growth factors (e.g., TGF-beta) or cytokines and chemokines, which promote survival, stemness and chemoradioresistance (Dzobo et al. 2023; Senthebane et al. 2017). In this regard, patient-derived cancer-associated fibroblasts (CAFs) have been shown to possess the ability to promote cisplatin resistance in cancer cells by paracrine effects. Changes in gene expression, including NANOG upregulation, mediated these effects (Peltanova et al. 2021). A recent study found that these cells were able to induce a transition of SCC-25 cells into paclitaxel-resistant cells by the paracrine action of IL-6 (Liu et al. 2021a, b). Also, “normal” keratinocytes present in the cancer field can contribute to stemness and chemoresistance induction. One of the mechanisms involved relies on the secretion of ligands that activate EGFR and/or FGFR receptors on cancer cells and promote the enrichment of CD44highSOX2high cells. This results in enhanced resistance to small molecule PI3K inhibition, which can be abolished by erlotinib (Nguyen et al. 2022). Thus, drug resistance cannot be considered as the property of isolated cancer cells but of the cell interactome characteristic of the tumor microenvironment (Dzobo et al. 2020). In addition, CAFs and TAMs are responsible for the increased production of the components of the extracellular matrix (ECM), which can contribute to chemoresistance (Dzobo et al. 2023; Senthebane et al. 2018). Indeed, ECM activated ERK and PI3K/Akt signaling in cancer cells and reduced sensitivity to cisplatin, fluorouracil and epirubicin, and the reduction in the level of collagen type I and fibronectin in ECM resulted in diminished colony formation of cancer cells and sensitization to cisplatin (Senthebane et al. 2018). Moreover, increased matrix stiffness, which was caused by increased deposition of fibrillar collagens and other proteins, together with enhanced matrix cross-linking, led to the stimulation of cell dormancy, and correlated with shorter relapse-free survival (Jingyuan et al. 2023).
The tumor microenvironment in solid tumors significantly contributes to immunosuppression (Dianat-Moghadam et al. 2022, 2023). Thus, despite the high prevalence of PD-L1 expression in HNSCC, the immune checkpoint inhibitors do not show satisfactory clinical response due to primary and adaptive resistance, including the immunosuppressive capabilities presented by CD44 + cells (Kok 2020). Cancer stem cells can affect immune cells by exosomes (Gonzalez-Callejo et al. 2023). Indeed, CSC-derived exosomes can mediate communication with other cells to protect the CSC-niche and to promote relapse (Gupta et al. 2021). Furthermore, CD44 + HNSCC cells were shown to secrete increased amounts of IL-8, granulocyte colony-stimulating factor, and TGF-β, leading to the inhibition of effector T or NK cells. In addition, CD44 + cells decreased the secretion of interferon gamma or IL-2 by peripheral blood mononuclear cells (Chikamatsu et al. 2011). All these effects may contribute to immune evasion in HNSCC.
Table 2 lists the chemicals that exhibit chemosensitizing effects by affecting HNSCC stem cells. Future research should focus on characterizing the clinical utility and optimizing the proposed strategies.
Conclusions
The current state of knowledge allows us to assume that HNSCC cancer stem cells are the most significant population of cells in the acquisition of drug resistance. Importantly, the stem cell hypothesis does not fully explain the occurrence of chemoresistance (Griso et al. 2022). For example, it has been shown that the pH reduction in tumors is responsible for cisplatin resistance because of drug entrapment in the acidic extracellular compartment. While microenvironment acidification could induce the expression of NANOG, CD44, and BMI1 in cells in vitro, it was shown that the restoration of physiological pH was sufficient for cell resensitization to cisplatin (de Bem Prunes et al. 2022). There is also a plethora of other mechanisms which can contribute to the resistant phenotype, but not all of them have been studied in relation to HNSCC stem cells (Griso et al. 2022).
Nevertheless, based on the current literature, the association between the stemness phenotype and chemoresistance in HNSCC is evident. Crucial aspects of this connection include the finding that chemotherapy leads to the enrichment of cancer stem cells (Basak et al. 2015; Bu et al. 2015; Jiang et al. 2018; Kim et al. 2017; Nakano et al. 2021; Nör et al. 2014; Subramanian et al. 2017, p. 90), drug-resistant cells show the enrichment of stem cell subpopulations (Kulsum et al. 2017; Lima de Oliveira et al. 2022; Murakami et al. 2022; Roy et al. 2018, 2019; Tsai et al. 2011), and isolated cancer stem cells are resistant to chemotherapy (Chen et al. 2010; Fernandes et al. 2022; Gunduz et al. 2019; Silva Galbiatti-Dias et al. 2018). In addition, various molecular mechanisms underlying stemness-related therapy resistance were identified (Barbato et al. 2019). The PI3K/Akt, Wnt/catenin, and Src pathways are all implicated, as are interleukin-induced STAT3 activation, epigenetic modulators, redox states, and the tumor microenvironment. Also metabolic reprogramming, one of the hallmarks of cancer, can contribute to stemness and chemoresistance. The activity of the enzymes associated with NAD + synthesis and consumption is frequently altered in cancer cells, including head and neck cancers (Togni et al. 2021). These aberrations may play a role in the acquisition of stemness potential (Novak Kujundžić et al. 2021). A recent study has shown that NAD + imbalance is characteristic of HNSCC stem cells. The targeting of NAD + biosynthetic pathways with the inhibitors of nicotinamide phosphoribosyltransferase (NAMPT) or nicotinate phosphoribosyltransferase (NAPRT) showed anti-tumor effects and exerted sensitization to docetaxel in xenograft mice. Moreover, the adaptive reboosting of NAD + synthesis by the upregulation of NAMPT or NAPRT, which was observed upon cell treatment, could be tackled by the combinatorial inhibition of both enzymes (Navas et al. 2023). This corroborates the importance of the use of mixes of chemicals to deal with the consequences of cell plasticity. Additionally, the isoenzymes of pyruvate dehydrogenase kinase (PDK1 and PDK2), which are associated with alterations of glucose metabolism called the Warburg effect, have been implicated in stemness and chemoresistance. Their knockdown led to the HNSCC sensitization to cisplatin and gemcitabine (Sun et al. 2022a, b). Interestingly, recent reports presented a new strategy for eradicating HNSCC stem cells by inducing their osteogenic differentiation (Jaksic Karisik et al. 2023; Patil et al. 2022).
Thus, there is a plethora of biological mechanisms responsible for stemness-induced chemoresistance and because of this very reason it is currently difficult to single out a target which would be best for the effective sensitization of stem cells in tumors (Yang et al. 2020). Perhaps, this would require some personalization using molecular diagnostic tests that have not yet been developed (Walcher et al. 2020). However, the difficulty in the selection of drug targets is also a consequence of high cellular plasticity (Salem and Salo 2023). Indeed, isolated subpopulations of stem cells were able to restore the original heterogenous cell populations (Navas et al. 2023). Thus, combinatorial sensitizing treatments may be the best option; however, more work is necessary to determine which compounds and targets show the highest synergistic potential. Furthermore, most of the currently available information was developed using selected cell lines and more research should be peformed in vivo, especially using patient-derived xenografts (or patient-derived organoids) or other relevant in vivo models (Salem and Salo 2023). Moreover, most in vivo studies used tumor size/volume as endpoint, but rarely analyzed cell subpopulations in the tumors, which would be helpful to prove that the observed effects are indeed dependent on the ablation of stem cells. Another factor that needs further elucidation is the sequence of treatments. Some evidence points to the utility of sequential treatments, with the stem cell-ablating chemical preceding the classical chemotheraputic dug. However, more evidence is necessary to find the best option. Thus, while the benefits of the pharmacological targeting of cancer stem cells by affecting various molecular targets were shown in vitro and in vivo, direct evidence of such benefits in HNSCC patients have not been documented so far, and the field needs well-designed relevant clinical studies which would test the clinical validity of the findings.
This paper corroborates targeting cancer stem cells as a promising strategy for overcoming therapy resistance in head and neck cancers. Chemicals aimed at stem cell ablation have shown adjuvant potential in animal studies, which warrants further research on the exact clinical utility of these stem cell-targeted strategies as chemosensitizers in humans.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Atashi F, Vahed N, Emamverdizadeh P, Fattahi S, Paya L (2021) Drug resistance against 5-fluorouracil and cisplatin in the treatment of head and neck squamous cell carcinoma: a systematic review. J Dental Res Dental Clin Dental Prospect 15(3):219–225. https://doi.org/10.34172/joddd.2021.036
Atashzar MR, Baharlou R, Karami J, Abdollahi H, Rezaei R, Pourramezan F, Zoljalali Moghaddam SH (2020) Cancer stem cells: a review from origin to therapeutic implications. J Cell Physiol 235(2):790–803. https://doi.org/10.1002/jcp.29044
Balermpas P, Rödel F, Liberz R, Oppermann J, Wagenblast J, Ghanaati S, Harter PN, Mittelbronn M, Weiss C, Rödel C, Fokas E (2014) Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer 111(8):1509–1518. https://doi.org/10.1038/bjc.2014.446
Barbato L, Bocchetti M, Di Biase A, Regad T (2019) Cancer stem cells and targeting strategies. Cells 8(8):926. https://doi.org/10.3390/cells8080926
Basak SK, Zinabadi A, Wu AW, Venkatesan N, Duarte VM, Kang JJ, Dalgard CL, Srivastava M, Sarkar FH, Wang MB, Srivatsan ES (2015) Liposome encapsulated curcumin-difluorinated (CDF) inhibits the growth of cisplatin resistant head and neck cancer stem cells. Oncotarget 6(21):18504–18517. https://doi.org/10.18632/oncotarget.4181
Bourguignon LYW, Wong G, Earle C, Chen L (2012) Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem 287(39):32800–32824. https://doi.org/10.1074/jbc.M111.308528
Bourguignon LYW, Wong G, Shiina M (2016) Up-regulation of histone methyltransferase, DOT1L, by matrix hyaluronan promotes microRNA-10 expression leading to tumor cell invasion and chemoresistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem 291(20):10571–10585. https://doi.org/10.1074/jbc.M115.700021
Boxberg M, Götz C, Haidari S, Dorfner C, Jesinghaus M, Drecoll E, Boskov M, Wolff KD, Weichert W, Haller B, Kolk A (2018) Immunohistochemical expression of CD44 in oral squamous cell carcinoma in relation to histomorphological parameters and clinicopathological factors. Histopathology 73(4):559–572. https://doi.org/10.1111/his.13496
Bu L-L, Zhao Z-L, Liu J-F, Ma S-R, Huang C-F, Liu B, Zhang W-F, Sun Z-J (2015) STAT3 blockade enhances the efficacy of conventional chemotherapeutic agents by eradicating head neck stemloid cancer cell. Oncotarget 6(39):41944–41958. https://doi.org/10.18632/oncotarget.5986
Chang C-W, Chen Y-S, Chou S-H, Han C-L, Chen Y-J, Yang C-C, Huang C-Y, Lo J-F (2014) Distinct subpopulations of head and neck cancer cells with different levels of intracellular reactive oxygen species exhibit diverse stemness, proliferation, and chemosensitivity. Can Res 74(21):6291–6305. https://doi.org/10.1158/0008-5472.CAN-14-0626
Chang M-T, Lee S-P, Fang C-Y, Hsieh P-L, Liao Y-W, Lu M-Y, Tsai L-L, Yu C-C, Liu C-M (2018) Chemosensitizing effect of honokiol in oral carcinoma stem cells via regulation of IL-6/Stat3 signaling. Environ Toxicol 33(11):1105–1112. https://doi.org/10.1002/tox.22587
Chen Y-W, Chen K-H, Huang P-I, Chen Y-C, Chiou G-Y, Lo W-L, Tseng L-M, Hsu H-S, Chang K-W, Chiou S-H (2010) Cucurbitacin I suppressed stem-like property and enhanced radiation-induced apoptosis in head and neck squamous carcinoma-derived CD44+ALDH1+ cells. Mol Cancer Ther 9(11):2879–2892. https://doi.org/10.1158/1535-7163.MCT-10-0504
Chen D, Wu M, Li Y, Chang I, Yuan Q, Ekimyan-Salvo M, Deng P, Yu B, Yu Y, Dong J, Szymanski JM, Ramadoss S, Li J, Wang C-Y (2017) Targeting BMI1 + cancer stem cells overcomes chemoresistance and inhibits metastases in squamous cell carcinoma. Cell Stem Cell 20(5):621-634.e6. https://doi.org/10.1016/j.stem.2017.02.003
Chen Y-J, You G-R, Lai M-Y, Lu L-S, Chen C-Y, Ting L-L, Lee H-L, Kanno Y, Chiou J-F, Cheng A-J (2020) A combined systemic strategy for overcoming cisplatin resistance in head and neck cancer: from target identification to drug discovery. Cancers 12(11):3482. https://doi.org/10.3390/cancers12113482
Chen T-M, Huang C-M, Hsieh M-S, Lin C-S, Lee W-H, Yeh C-T, Liu S-C (2022) TRPM7 via calcineurin/NFAT pathway mediates metastasis and chemotherapeutic resistance in head and neck squamous cell carcinoma. Aging 14(12):5250–5270. https://doi.org/10.18632/aging.204154
Chikamatsu K, Takahashi G, Sakakura K, Ferrone S, Masuyama K (2011) Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head Neck 33(2):208–215. https://doi.org/10.1002/hed.21420
Chikamatsu K, Ishii H, Takahashi G, Okamoto A, Moriyama M, Sakakura K, Masuyama K (2012) Resistance to apoptosis-inducing stimuli in CD44+ head and neck squamous cell carcinoma cells. Head Neck 34(3):336–343. https://doi.org/10.1002/hed.21732
Cirillo N, Wu C, Prime SS (2021) Heterogeneity of cancer stem cells in tumorigenesis, metastasis, and resistance to antineoplastic treatment of head and neck tumours. Cells 10(11):3068. https://doi.org/10.3390/cells10113068
de Bem Prunes B, Nunes JS, da Silva VP, Laureano NK, Gonçalves DR, Machado IS, Barbosa S, Lamers ML, Rados PV, Kurth I, Hess J, Jou A, Visioli F (2022) The role of tumor acidification in aggressiveness, cell dissemination and treatment resistance of oral squamous cell carcinoma. Life Sci 288:120163. https://doi.org/10.1016/j.lfs.2021.120163
Dianat-Moghadam H, Mahari A, Salahlou R, Khalili M, Azizi M, Sadeghzadeh H (2022) Immune evader cancer stem cells direct the perspective approaches to cancer immunotherapy. Stem Cell Res Ther 13(1):150. https://doi.org/10.1186/s13287-022-02829-9
Dianat-Moghadam H, Sharifi M, Salehi R, Keshavarz M, Shahgolzari M, Amoozgar Z (2023) Engaging stemness improves cancer immunotherapy. Cancer Lett 554:216007. https://doi.org/10.1016/j.canlet.2022.216007
Dong J, Li J, Li Y, Ma Z, Yu Y, Wang C-Y (2021) Transcriptional super-enhancers control cancer stemness and metastasis genes in squamous cell carcinoma. Nat Commun 12(1):3974. https://doi.org/10.1038/s41467-021-24137-1
Dzobo K (2020) Taking a full snapshot of cancer biology: deciphering the tumor microenvironment for effective cancer therapy in the oncology clinic. OMICS 24(4):175–179. https://doi.org/10.1089/omi.2020.0019
Dzobo K, Senthebane DA, Ganz C, Thomford NE, Wonkam A, Dandara C (2020) Advances in therapeutic targeting of cancer stem cells within the tumor microenvironment: an updated review. Cells 9(8):1896. https://doi.org/10.3390/cells9081896
Dzobo K, Senthebane DA, Dandara C (2023) The tumor microenvironment in tumorigenesis and therapy resistance revisited. Cancers 15(2):376. https://doi.org/10.3390/cancers15020376
Elkashty OA, Tran SD (2020) Broccoli extract increases drug-mediated cytotoxicity towards cancer stem cells of head and neck squamous cell carcinoma. Br J Cancer 123(9):1395–1403. https://doi.org/10.1038/s41416-020-1025-1
Elkashty OA, Abu Elghanam G, Su X, Liu Y, Chauvin PJ, Tran SD (2020) Cancer stem cells enrichment with surface markers CD271 and CD44 in human head and neck squamous cell carcinomas. Carcinogenesis 41(4):458–466. https://doi.org/10.1093/carcin/bgz182
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F (2019) Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144(8):1941–1953. https://doi.org/10.1002/ijc.31937
Fernandes GMM, Serafim Junior V, Galbiatti-Dias ALS, Ferreira LAM, Castanhole-Nunes MMU, Kawasaki-Oyama RS, Maniglia JV, Pavarino EC, Goloni-Bertollo EM (2022) Treatment effects of the EGFR pathway drugs on head and neck cancer stem cells. Am J Cancer Res 12(9):4196–4210
Fukusumi T, Ishii H, Konno M, Yasui T, Nakahara S, Takenaka Y, Yamamoto Y, Nishikawa S, Kano Y, Ogawa H, Hasegawa S, Hamabe A, Haraguchi N, Doki Y, Mori M, Inohara H (2014) CD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinoma. Br J Cancer 111(3):506–514. https://doi.org/10.1038/bjc.2014.289
Garcia-Mayea Y, Mir C, Muñoz L, Benavente S, Castellvi J, Temprana J, Maggio V, Lorente J, Paciucci R, Leonart MEL (2019) Autophagy inhibition as a promising therapeutic target for laryngeal cancer. Carcinogenesis. https://doi.org/10.1093/carcin/bgz080
Garcia-Mayea Y, Mir C, Carballo L, Castellvi J, Temprana-Salvador J, Lorente J, Benavente S, García-Pedrero JM, Allonca E, Rodrigo JP, Leonart MEL (2020) TSPAN1: a novel protein involved in head and neck squamous cell carcinoma chemoresistance. Cancers 12(11):3269. https://doi.org/10.3390/cancers12113269
Gomez KE, Wu F, Keysar SB, Morton JJ, Miller B, Chimed T-S, Le PN, Nieto C, Chowdhury FN, Tyagi A, Lyons TR, Young CD, Zhou H, Somerset HL, Wang X-J, Jimeno A (2020) Cancer cell CD44 mediates macrophage/monocyte-driven regulation of head and neck cancer stem cells. Can Res 80(19):4185–4198. https://doi.org/10.1158/0008-5472.CAN-20-1079
Gonzalez-Callejo P, Guo Z, Ziglari T, Claudio NM, Nguyen KH, Oshimori N, Seras-Franzoso J, Pucci F (2023) Cancer stem cell-derived extracellular vesicles preferentially target MHC-II-macrophages and PD1+ T cells in the tumor microenvironment. PLoS ONE 18(2):e0279400. https://doi.org/10.1371/journal.pone.0279400
Griso AB, Acero-Riaguas L, Castelo B, Cebrián-Carretero JL, Sastre-Perona A (2022) Mechanisms of cisplatin resistance in HPV negative head and neck squamous cell carcinomas. Cells 11(3):561. https://doi.org/10.3390/cells11030561
Guan G-F, Tang X-X, Zhang D-J, Zheng Y, Yu D-J, Zhao Y, Lu Y-Q, Zhu L (2015) Constitutive secretion of Interleukin-4 dictates CD133+ side population cells to resist drug treatment and cell death. J BUON 20(5):1350–1359
Gunduz M, Gunduz E, Tamagawa S, Enomoto K, Hotomi M (2019) Identification and chemoresistance of cancer stem cells in HPV-negative oropharyngeal cancer. Oncol Lett 19(1):965–971. https://doi.org/10.3892/ol.2019.11127
Gupta S, Kumar P, Das BC (2021) HPV+ve/−ve oral-tongue cancer stem cells: a potential target for relapse-free therapy. Transl Oncol 14(1):100919. https://doi.org/10.1016/j.tranon.2020.100919
Gupta V, Maurya MK, Agarwal P, Kumar M, Sagar M, Raghuvanshi S, Gupta S (2022) Expression of aldehyde dehydrogenase 1A1 in oral squamous cell carcinoma and its correlation with clinicopathological parameters. Natl J Maxillofac Surg 13(2):208–215. https://doi.org/10.4103/njms.njms_402_21
Guy J-B, Espenel S, Louati S, Gauthier A, Garcia M-A, Vial N, Malésys C, Ardail D, Alphonse G, Wozny A-S, Rodriguez-Lafrasse C, Magné N (2021) Combining radiation to EGFR and Bcl-2 blockade: a new approach to target cancer stem cells in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol 147(7):1905–1916. https://doi.org/10.1007/s00432-021-03593-8
Han Y, Xu S, Ye W, Wang Y, Zhang X, Deng J, Zhang Z, Liu L, Liu S (2021) Targeting LSD1 suppresses stem cell-like properties and sensitizes head and neck squamous cell carcinoma to PD-1 blockade. Cell Death Dis 12(11):993. https://doi.org/10.1038/s41419-021-04297-0
Hermida-Prado F, Villaronga MÁ, Granda-Díaz R, del-Río-Ibisate N, Santos L, Hermosilla MA, Oro P, Allonca E, Agorreta J, Garmendia I, Tornín J, Perez-Escuredo J, Fuente R, Montuenga LM, Morís F, Rodrigo JP, Rodríguez R, García-Pedrero JM (2019) The SRC inhibitor dasatinib induces stem cell-like properties in head and neck cancer cells that are effectively counteracted by the mithralog EC-8042. J Clin Med 8(8):1157. https://doi.org/10.3390/jcm8081157
Herzog AE, Warner KA, Zhang Z, Bellile E, Bhagat MA, Castilho RM, Wolf GT, Polverini PJ, Pearson AT, Nör JE (2021) The IL-6R and Bmi-1 axis controls self-renewal and chemoresistance of head and neck cancer stem cells. Cell Death Dis 12(11):988. https://doi.org/10.1038/s41419-021-04268-5
Hsueh W-T, Chen S-H, Chien C-H, Chou S-W, Chi P-I, Chu J-M, Chang K-Y (2021) SOD2 enhancement by long-term inhibition of the PI3K pathway confers multi-drug resistance and enhanced tumor-initiating features in head and neck cancer. Int J Mol Sci 22(20):11260. https://doi.org/10.3390/ijms222011260
Huang C, Yoon C, Zhou X-H, Zhou Y-C, Zhou W-W, Liu H, Yang X, Lu J, Lee SY, Huang K (2020a) ERK1/2-Nanog signaling pathway enhances CD44(+) cancer stem-like cell phenotypes and epithelial-to-mesenchymal transition in head and neck squamous cell carcinomas. Cell Death Dis 11(4):266. https://doi.org/10.1038/s41419-020-2448-6
Huang T, Song X, Xu D, Tiek D, Goenka A, Wu B, Sastry N, Hu B, Cheng S-Y (2020b) Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics 10(19):8721–8743. https://doi.org/10.7150/thno.41648
Imai T, Tamai K, Oizumi S, Oyama K, Yamaguchi K, Sato I, Satoh K, Matsuura K, Saijo S, Sugamura K, Tanaka N (2013) CD271 defines a stem cell-like population in hypopharyngeal cancer. PLoS One 8(4):e62002. https://doi.org/10.1371/journal.pone.0062002
Jaksic Karisik M, Lazarevic M, Mitic D, Nikolic N, Milosevic Markovic M, Jelovac D, Milasin J (2023) Osteogenic and adipogenic differentiation potential of oral cancer stem cells may offer new treatment modalities. Int J Mol Sci 24(5):4704. https://doi.org/10.3390/ijms24054704
Jang T-H, Huang W-C, Tung S-L, Lin S-C, Chen P-M, Cho C-Y, Yang Y-Y, Yen T-C, Lo G-H, Chuang S-E, Wang L-H (2022) MicroRNA-485-5p targets keratin 17 to regulate oral cancer stemness and chemoresistance via the integrin/FAK/Src/ERK/β-catenin pathway. J Biomed Sci 29(1):42. https://doi.org/10.1186/s12929-022-00824-z
Jiang P, Xu C, Zhou M, Zhou H, Dong W, Wu X, Chen A, Feng Q (2018) RXRα-enriched cancer stem cell-like properties triggered by CDDP in head and neck squamous cell carcinoma (HNSCC). Carcinogenesis 39(2):252–262. https://doi.org/10.1093/carcin/bgx138
Jingyuan L, Yu L, Hong J, Tao W, Kan L, Xiaomei L, Guiqing L, Yujie L (2023) Matrix stiffness induces an invasive-dormant subpopulation via cGAS-STING axis in oral cancer. Transl Oncol 33:101681. https://doi.org/10.1016/j.tranon.2023.101681
Kashyap T, Pramanik KK, Nath N, Mishra P, Singh AK, Nagini S, Rana A, Mishra R (2018) Crosstalk between Raf-MEK-ERK and PI3K-Akt-GSK3β signaling networks promotes chemoresistance, invasion/migration and stemness via expression of CD44 variants (v4 and v6) in oral cancer. Oral Oncol 86:234–243. https://doi.org/10.1016/j.oraloncology.2018.09.028
Kashyap T, Nath N, Mishra P, Jha A, Nagini S, Mishra R (2020) Pluripotency transcription factor Nanog and its association with overall oral squamous cell carcinoma progression, cisplatin-resistance, invasion and stemness acquisition. Head Neck 42(11):3282–3294. https://doi.org/10.1002/hed.26373
Keysar SB, Le PN, Miller B, Jackson BC, Eagles JR, Nieto C, Kim J, Tang B, Glogowska MJ, Morton JJ, Padilla-Just N, Gomez K, Warnock E, Reisinger J, Arcaroli JJ, Messersmith WA, Wakefield LM, Gao D, Tan A-C, Jimeno A (2017) Regulation of head and neck squamous cancer stem cells by PI3K and SOX2. J Natl Cancer Inst 109(1):djw189. https://doi.org/10.1093/jnci/djw189
Kim J, Shin JH, Chen C-H, Cruz L, Farnebo L, Yang J, Borges P, Kang G, Mochly-Rosen D, Sunwoo JB (2017) Targeting aldehyde dehydrogenase activity in head and neck squamous cell carcinoma with a novel small molecule inhibitor. Oncotarget 8(32):52345–52356. https://doi.org/10.18632/oncotarget.17017
Kim SI, Woo SR, Noh JK, Lee MK, Lee YC, Lee JW, Kong M, Ko S-G, Eun Y-G (2022) Association between cancer stem cell gene expression signatures and prognosis in head and neck squamous cell carcinoma. BMC Cancer 22(1):1077. https://doi.org/10.1186/s12885-022-10184-4
Kok VC (2020) Current understanding of the mechanisms underlying immune evasion from PD-1/PD-L1 immune checkpoint blockade in head and neck cancer. Front Oncol 10:268. https://doi.org/10.3389/fonc.2020.00268
Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nör JE (2010) Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Can Res 70(23):9969–9978. https://doi.org/10.1158/0008-5472.CAN-10-1712
Kulsum S, Sudheendra HV, Pandian R, Ravindra DR, Siddappa G, Nisheena R, Chevour P, Ramachandran B, Sagar M, Jayaprakash A, Mehta A, Kekatpure V, Hedne N, Kuriakose MA, Suresh A (2017) Cancer stem cell mediated acquired chemoresistance in head and neck cancer can be abrogated by aldehyde dehydrogenase 1 A1 inhibition: cancer stem cells in drug resistance. Mol Carcinog 56(2):694–711. https://doi.org/10.1002/mc.22526
Kuşoğlu A, Biray Avcı Ç (2019) Cancer stem cells: a brief review of the current status. Gene 681:80–85. https://doi.org/10.1016/j.gene.2018.09.052
La Fleur L, Johansson A-C, Roberg K (2012) A CD44high/EGFRlow subpopulation within head and neck cancer cell lines shows an epithelial-mesenchymal transition phenotype and resistance to treatment. PLoS One 7(9):e44071. https://doi.org/10.1371/journal.pone.0044071
Lee SH, Nam HJ, Kang HJ, Samuels TL, Johnston N, Lim YC (2015) Valproic acid suppresses the self-renewal and proliferation of head and neck cancer stem cells. Oncol Rep 34(4):2065–2071. https://doi.org/10.3892/or.2015.4145
Lee C-R, Lee SH, Rigas NK, Kim RH, Kang MK, Park N-H, Shin K-H (2016) Elevated expression of JMJD6 is associated with oral carcinogenesis and maintains cancer stemness properties. Carcinogenesis 37(2):119–128. https://doi.org/10.1093/carcin/bgv169
Li Z, Wu X, Li J, Yu S, Ke X, Yan T, Zhu Y, Cheng J, Yang J (2022) HMGA2-Snai2 axis regulates tumorigenicity and stemness of head and neck squamous cell carcinoma. Exp Cell Res 418(1):113271. https://doi.org/10.1016/j.yexcr.2022.113271
Lim YC, Kang HJ, Moon JH (2014) C-Met pathway promotes self-renewal and tumorigenecity of head and neck squamous cell carcinoma stem-like cell. Oral Oncol 50(7):633–639. https://doi.org/10.1016/j.oraloncology.2014.04.004
Lima de Oliveira J, Moré Milan T, Longo Bighetti-Trevisan R, Fernandes RR, Machado Leopoldino A, Oliveira de Almeida L (2022) Epithelial–mesenchymal transition and cancer stem cells: a route to acquired cisplatin resistance through epigenetics in HNSCC. Oral Dis 29(5):1991–2005. https://doi.org/10.1111/odi.14209
Liu C, Billet S, Choudhury D, Cheng R, Haldar S, Fernandez A, Biondi S, Liu Z, Zhou H, Bhowmick NA (2021a) Bone marrow mesenchymal stem cells interact with head and neck squamous cell carcinoma cells to promote cancer progression and drug resistance. Neoplasia 23(1):118–128. https://doi.org/10.1016/j.neo.2020.11.012
Liu S-C, Wu Y-C, Huang C-M, Hsieh M-S, Huang T-Y, Huang C-S, Hsu T-N, Huang M-S, Lee W-H, Yeh C-T, Lin C-S (2021b) Inhibition of Bruton’s tyrosine kinase as a therapeutic strategy for chemoresistant oral squamous cell carcinoma and potential suppression of cancer stemness. Oncogenesis 10(2):20. https://doi.org/10.1038/s41389-021-00308-z
Lu B-C, Li J, Yu W-F, Zhang G-Z, Wang H-M, Ma H-M (2016) Elevated expression of Nrf2 mediates multidrug resistance in CD133+ head and neck squamous cell carcinoma stem cells. Oncol Lett 12(6):4333–4338. https://doi.org/10.3892/ol.2016.5269
Macha MA, Rachagani S, Qazi AK, Jahan R, Gupta S, Patel A, Seshacharyulu P, Lin C, Li S, Wang S, Verma V, Kishida S, Kishida M, Nakamura N, Kibe T, Lydiatt WM, Smith RB, Ganti AK, Jones DT, Jain M (2017) Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget 8(13):20961–20973. https://doi.org/10.18632/oncotarget.15468
Milan TM, Eskenazi APE, de Oliveira LD, da Silva G, Bighetti-Trevisan RL, Freitas GP, Almeida LO (2023) Interplay between EZH2/β-catenin in stemness of cisplatin-resistant HNSCC and their role as therapeutic targets. Cell Signall 109:110773. https://doi.org/10.1016/j.cellsig.2023.110773
Mir C, Garcia-Mayea Y, Garcia L, Herrero P, Canela N, Tabernero R, Lorente J, Castellvi J, Allonca E, García-Pedrero J, Rodrigo JP, Carracedo Á, LLeonart ME (2021) SDCBP modulates stemness and chemoresistance in head and neck squamous cell carcinoma through Src activation. Cancers 13(19):4952. https://doi.org/10.3390/cancers13194952
Mishra S, Tiwari V, Arora A, Gupta S, Anand N, Husain N (2020) Increased expression of Oct4, Nanog and CD24 predicts poor response to chemo-radiotherapy and unfavourable prognosis in locally advanced oral squamous cell carcinoma. Asian Pacific J Cancer Prevent 21(9):2539–2547. https://doi.org/10.31557/APJCP.2020.21.9.2539
Modur V, Joshi P, Nie D, Robbins KT, Khan AU, Rao K (2016) CD24 expression may play a role as a predictive indicator and a modulator of cisplatin treatment response in head and neck squamous cellular carcinoma. Plos one 11(6):e0156651. https://doi.org/10.1371/journal.pone.0156651
Mudra SE, Sadhukhan P, Ugurlu MT, Alam S, Hoque MO (2021) Therapeutic targeting of cancer stem cells in lung, head and neck, and bladder cancers. Cancers 13(20):5098. https://doi.org/10.3390/cancers13205098
Murakami K, Umemura N, Adachi M, Motoki M, Ohkoshi E (2022) ABCG2, CD44 and SOX9 are increased with the acquisition of drug resistance and involved in cancer stem cell activities in head and neck squamous cell carcinoma cells. Exp Ther Med 24(6):722. https://doi.org/10.3892/etm.2022.11658
Murillo-Sauca O, Chung MK, Shin JH, Karamboulas C, Kwok S, Jung YH, Oakley R, Tysome JR, Farnebo LO, Kaplan MJ, Sirjani D, Divi V, Holsinger FC, Tomeh C, Nichols A, Le QT, Colevas AD, Kong CS, Uppaluri R, Sunwoo JB (2014) CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget 5(16):6854–6866. https://doi.org/10.18632/oncotarget.2269
Naik PP, Mukhopadhyay S, Panda PK, Sinha N, Das CK, Mishra R, Patil S, Bhutia SK (2018) Autophagy regulates cisplatin-induced stemness and chemoresistance via the upregulation of CD44, ABCB1 and ADAM17 in oral squamous cell carcinoma. Cell Prolif 51(1):e12411. https://doi.org/10.1111/cpr.12411
Nakano T, Warner KA, Oklejas AE, Zhang Z, Rodriguez-Ramirez C, Shuman AG, Nör JE (2021) MTOR inhibition ablates cisplatin-resistant salivary gland cancer stem cells. J Dent Res 100(4):377–386. https://doi.org/10.1177/0022034520965141
Navas LE, Blanco-Alcaina E, Suarez-Martinez E, Verdugo-Sivianes EM, Espinosa-Sanchez A, Sanchez-Diaz L, Dominguez-Medina E, Fernandez-Rozadilla C, Carracedo A, Wu LE, Carnero A (2023) NAD pool as an antitumor target against cancer stem cells in head and neck cancer. J Exp Clin Cancer Res 42(1):55. https://doi.org/10.1186/s13046-023-02631-2
Nguyen KA, Keith MJ, Keysar SB, Hall SC, Bimali A, Jimeno A, Wang X, Young CD (2022) Epidermal growth factor receptor signaling in precancerous keratinocytes promotes neighboring head and neck cancer squamous cell carcinoma cancer stem cell-like properties and phosphoinositide 3-kinase inhibitor insensitivity. Mol Carcinog 61(7):664–676. https://doi.org/10.1002/mc.23409
Nör C, Zhang Z, Warner KA, Bernardi L, Visioli F, Helman JI, Roesler R, Nör JE (2014) Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia 16(2):137-W8. https://doi.org/10.1593/neo.131744
Novak Kujundžić R, Prpić M, Đaković N, Dabelić N, Tomljanović M, Mojzeš A, Fröbe A, Trošelj KG (2021) Nicotinamide N-methyltransferase in acquisition of stem cell properties and therapy resistance in cancer. Int J Mol Sci 22(11):5681. https://doi.org/10.3390/ijms22115681
Ortiz RC, Lopes NM, Amôr NG, Ponce JB, Schmerling CK, Lara VS, Moyses RA, Rodini CO (2018) CD44 and ALDH1 immunoexpression as prognostic indicators of invasion and metastasis in oral squamous cell carcinoma. J Oral Pathol Med 47(8):740–747. https://doi.org/10.1111/jop.12734
Osazuwa-Peters N, Simpson MC, Zhao L, Boakye EA, Olomukoro SI, Deshields T, Loux TM, Varvares MA, Schootman M (2018) Suicide risk among cancer survivors: head and neck versus other cancers. Cancer 124(20):4072–4079. https://doi.org/10.1002/cncr.31675
Ota N, Ohno J, Seno K, Taniguchi K, Ozeki S (2014) In vitro and in vivo expression of aldehyde dehydrogenase 1 in oral squamous cell carcinoma. Int J Oncol 44(2):435–442. https://doi.org/10.3892/ijo.2013.2188
Pai S, Yadav VK, Kuo K-T, Pikatan NW, Lin C-S, Chien M-H, Lee W-H, Hsiao M, Chiu S-C, Yeh C-T, Tsai J-T (2021) PDK1 Inhibitor BX795 improves cisplatin and radio-efficacy in oral squamous cell carcinoma by downregulating the PDK1/CD47/Akt-mediated glycolysis signaling pathway. Int J Mol Sci 22(21):11492. https://doi.org/10.3390/ijms222111492
Paluszczak J (2020) The significance of the dysregulation of canonical Wnt signaling in head and neck squamous cell carcinomas. Cells 9(3):723. https://doi.org/10.3390/cells9030723
Patil S, Al-Brakati A, Abidi NH, Almasri MA, Almeslet AS, Patil VR, Raj AT, Bhandi S (2022) CD44-positive cancer stem cells from oral squamous cell carcinoma exhibit reduced proliferation and stemness gene expression upon adipogenic induction. Med Oncol (northwood, London, England) 39(2):23. https://doi.org/10.1007/s12032-021-01617-4
Peltanova B, Liskova M, Gumulec J, Raudenska M, Polanska HH, Vaculovic T, Kalfert D, Grega M, Plzak J, Betka J, Masarik M (2021) Sensitivity to cisplatin in head and neck cancer cells is significantly affected by patient-derived cancer-associated fibroblasts. Int J Mol Sci 22(4):1912. https://doi.org/10.3390/ijms22041912
Peng C-Y, Wang T-Y, Lee S-S, Hsieh P-L, Liao Y-W, Tsai L-L, Fang C-Y, Yu C-C, Hsieh C-S (2018) Let-7c restores radiosensitivity and chemosensitivity and impairs stemness in oral cancer cells through inhibiting interleukin-8. J Oral Pathol Med 47(6):590–597. https://doi.org/10.1111/jop.12711
Peng C-Y, Yu C-C, Huang C-C, Liao Y-W, Hsieh P-L, Chu P-M, Yu C-H, Lin S-S (2022) Magnolol inhibits cancer stemness and IL-6/Stat3 signaling in oral carcinomas. J Formos Med Assoc 121(1):51–57. https://doi.org/10.1016/j.jfma.2021.01.009
Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE (2007) Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 104(3):973–978. https://doi.org/10.1073/pnas.0610117104
Pu Y, Li Q, Wang Y, Xu L, Qiao Q, Guo Y, Guo C (2021) PERK-mediated IL8 secretion can enhance the migration, invasion, and cisplatin resistance of CD10-positive oral cancer cells. BMC Cancer 21(1):1283. https://doi.org/10.1186/s12885-021-09025-7
Roy S, Roy S, Kar M, Padhi S, Saha A, Anuja K, Banerjee B (2018) Role of p38 MAPK in disease relapse and therapeutic resistance by maintenance of cancer stem cells in head and neck squamous cell carcinoma. J Oral Pathol Med 47(5):492–501. https://doi.org/10.1111/jop.12707
Roy S, Roy S, Kar M, Chakraborty A, Kumar A, Delogu F, Asthana S, Hande MP, Banerjee B (2019) Combined treatment with cisplatin and the tankyrase inhibitor XAV-939 increases cytotoxicity, abrogates cancer-stem-like cell phenotype and increases chemosensitivity of head-and-neck squamous-cell carcinoma cells. Mutat Res/genet Toxicol Environ Mutagen 846:503084. https://doi.org/10.1016/j.mrgentox.2019.503084
Roy S, Roy S, Anuja K, Thakur S, Akhter Y, Padhi S, Banerjee B (2021) P38 Mitogen-activated protein kinase modulates cisplatin resistance in head and neck squamous cell carcinoma cells. Arch Oral Biol 122:104981. https://doi.org/10.1016/j.archoralbio.2020.104981
Salem A, Salo T (2023) Identity matters: cancer stem cells and tumour plasticity in head and neck squamous cell carcinoma. Expert Rev Mol Med 25:e8. https://doi.org/10.1017/erm.2023.4
Senthebane DA, Rowe A, Thomford NE, Shipanga H, Munro D, Mazeedi MAMA, Almazyadi HAM, Kallmeyer K, Dandara C, Pepper MS, Parker MI, Dzobo K (2017) The role of tumor microenvironment in chemoresistance: to survive, keep your enemies closer. Int J Mol Sci 18(7):1586. https://doi.org/10.3390/ijms18071586
Senthebane D, Jonker T, Rowe A, Thomford N, Munro D, Dandara C, Wonkam A, Govender D, Calder B, Soares N, Blackburn J, Parker M, Dzobo K (2018) The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. Int J Mol Sci 19(10):2861. https://doi.org/10.3390/ijms19102861
Sheng X, Li Y, Li Y, Liu W, Lu Z, Zhan J, Xu M, Chen L, Luo X, Cai G, Zhang S (2019) PLOD2 contributes to drug resistance in laryngeal cancer by promoting cancer stem cell-like characteristics. BMC Cancer 19(1):840. https://doi.org/10.1186/s12885-019-6029-y
Shigeishi H, Yokoyama S, Murodumi H, Sakuma M, Fukada S, Okuda S, Yamakado N, Ono S, Takechi M, Ohta K (2022) Melatonin enhances cisplatin-induced cell death through inhibition of DERL1 in mesenchymal-like CD44high OSCC cells. J Oral Pathol Med 51(3):281–289. https://doi.org/10.1111/jop.13242
Silva Galbiatti-Dias AL, Fernandes GMM, Castanhole-Nunes MMU, Hidalgo LF, Nascimento Filho CHV, Kawasaki-Oyama RS, Ferreira LAM, Biselli-Chicote PM, Pavarino ÉC, Goloni-Bertollo EM (2018) Relationship between CD44high/CD133high/CD117high cancer stem cells phenotype and Cetuximab and Paclitaxel treatment response in head and neck cancer cell lines. Am J Cancer Res 8(8):1633–1641
Sinnung S, Janvilisri T, Kiatwuthinon P (2021) Reversal of cisplatin sensitization and abrogation of cisplatin-enriched cancer stem cells in 5–8F nasopharyngeal carcinoma cell line through a suppression of Wnt/β-catenin-signaling pathway. Mol Cell Biochem 476(4):1663–1672. https://doi.org/10.1007/s11010-020-04045-6
Siqueira JM, Heguedusch D, Rodini CO, Nunes FD, Rodrigues MFSD (2023) Mechanisms involved in cancer stem cell resistance in head and neck squamous cell carcinoma. Cancer Drug Resist 6(1):116–137. https://doi.org/10.20517/cdr.2022.107
Sola AM, Johnson DE, Grandis JR (2019) Investigational multitargeted kinase inhibitors in development for head and neck neoplasms. Expert Opin Investig Drugs 28(4):351–363. https://doi.org/10.1080/13543784.2019.1581172
Song M, Liu X, Li T, Zhang Y, Zhao X, Sun W, Li Z (2022) Silencing PLOD2 attenuates cancer stem cell-like characteristics and cisplatin-resistant through Integrin β1 in laryngeal cancer. Transl Oncol 22:101460. https://doi.org/10.1016/j.tranon.2022.101460
Subramanian C, Kovatch KJ, Sim MW, Wang G, Prince ME, Carey TE, Davis R, Blagg BSJ, Cohen MS (2017) Novel C-Terminal heat shock protein 90 inhibitors (KU711 and Ku757) are effective in targeting head and neck squamous cell carcinoma cancer stem cells. Neoplasia 19(12):1003–1011. https://doi.org/10.1016/j.neo.2017.09.003
Sun W-H, Chen Y-H, Lee H-H, Tang Y-W, Sun K-H (2022a) PDK1- and PDK2-mediated metabolic reprogramming contributes to the TGFβ1-promoted stem-like properties in head and neck cancer. Cancer Metab 10(1):23. https://doi.org/10.1186/s40170-022-00300-0
Sun W-H, Peng T-J, Tang S-J, Lin J-Y, Wang C-Y, Fang H-J, Sun K-H (2022b) CXCR3 isoform A promotes head and neck cancer progression by enhancing stem-like property and chemoresistance. J Oral Pathol Med 51(9):791–800. https://doi.org/10.1111/jop.13346
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Tavares MO, Milan TM, Bighetti-Trevisan RL, Leopoldino AM, de Almeida LO (2022) Pharmacological inhibition of HDAC6 overcomes cisplatin chemoresistance by targeting cancer stem cells in oral squamous cell carcinoma. J Oral Pathol Med 51(6):529–537. https://doi.org/10.1111/jop.13326
Togni L, Mascitti M, Sartini D, Campagna R, Pozzi V, Salvolini E, Offidani A, Santarelli A, Emanuelli M (2021) Nicotinamide N-methyltransferase in head and neck tumors: a comprehensive review. Biomolecules 11(11):1594. https://doi.org/10.3390/biom11111594
Tsai L-L, Yu C-C, Chang Y-C, Yu C-H, Chou M-Y (2011) Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma: stemness markers and chemo-resistance. J Oral Pathol Med 40(8):621–628. https://doi.org/10.1111/j.1600-0714.2011.01015.x
Vipparthi K, Hari K, Chakraborty P, Ghosh S, Patel AK, Ghosh A, Biswas NK, Sharan R, Arun P, Jolly MK, Singh S (2022) Emergence of hybrid states of stem-like cancer cells correlates with poor prognosis in oral cancer. Iscience 25(5):104317. https://doi.org/10.1016/j.isci.2022.104317
Walcher L, Kistenmacher A-K, Suo H, Kitte R, Dluczek S, Strauß A, Blaudszun A-R, Yevsa T, Fricke S, Kossatz-Boehlert U (2020) Cancer stem cells—origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol 11:1280. https://doi.org/10.3389/fimmu.2020.01280
Wang W, Yi M, Chen S, Li J, Zhang H, Xiong W, Li G, Li X, Xiang B (2017) NOR1 suppresses cancer stem-like cells properties of tumor cells via the inhibition of the AKT-GSK-3β-Wnt/β-catenin-ALDH1A1 signal circuit. J Cell Physiol 232(10):2829–2840. https://doi.org/10.1002/jcp.25706
Wang W, Li X, Wang F, Sun X (2018) Effect of TET1 regulating MGMT on chemotherapy resistance of oral squamous cell carcinoma stem cells. J Cell Biochem 119(1):723–735. https://doi.org/10.1002/jcb.26236
Wang Y, Li Q, Xu L, Chen J, Pu Y, Wang L, Sun H, Guo Y, Guo C (2021) Cancer stemness of CD10-positive cells regulated by Hedgehog pathway promotes the resistance to cisplatin in oral squamous cell carcinoma. Oral Dis 27(6):1403–1411. https://doi.org/10.1111/odi.13673
White AC, Lowry WE (2015) Refining the role for adult stem cells as cancer cells of origin. Trends Cell Biol 25(1):11–20. https://doi.org/10.1016/j.tcb.2014.08.008
Wu Y, Wu PY (2009) CD133 as a marker for cancer stem cells: progresses and concerns. Stem Cells Dev 18(8):1127–1134. https://doi.org/10.1089/scd.2008.0338
Wu J, Mu Q, Thiviyanathan V, Annapragada A, Vigneswaran N (2014) Cancer stem cells are enriched in Fanconi anemia head and neck squamous cell carcinomas. Int J Oncol 45(6):2365–2372. https://doi.org/10.3892/ijo.2014.2677
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H (2020) Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther 5(1):8. https://doi.org/10.1038/s41392-020-0110-5
Yin W, Wang J, Jiang L, James Kang Y (2021) Cancer and stem cells. Exp Biol Med (maywood, N.J.) 246(16):1791–1801. https://doi.org/10.1177/15353702211005390
Yokoyama S, Shigeishi H, Murodumi H, Sakuma M, Ono S, Tobiume K, Ohta K, Takechi M (2021) Effects of miR-224–5p-enhanced downregulation of pannexin-1 on docetaxel-induced apoptosis in amoeboid-like CD44high oral cancer cells. Eur J Oral Sci 129(5):e12812. https://doi.org/10.1111/eos.12812
Yu SS, Cirillo N (2020) The molecular markers of cancer stem cells in head and neck tumors. J Cell Physiol 235(1):65–73. https://doi.org/10.1002/jcp.28963
Yu C-H, Yu C-C (2014) Photodynamic Therapy with 5-Aminolevulinic acid (ALA) impairs tumor initiating and chemo-resistance property in head and neck cancer-derived cancer stem cells. PLoS One 9(1):e87129. https://doi.org/10.1371/journal.pone.0087129
Zhao X, Shu D, Sun W, Si S, Ran W, Guo B, Cui L (2022) PLEK2 promotes cancer stemness and tumorigenesis of head and neck squamous cell carcinoma via the c-Myc-mediated positive feedback loop. Cancer Commun 42(10):987–1007. https://doi.org/10.1002/cac2.12349
Funding
This research was funded by The National Science Centre, Poland, grant number 2022/45/N/NZ7/01780.
Author information
Authors and Affiliations
Contributions
JP and DD conceived the idea for the review article, performed the literature search and drafted the manuscript, and all authors commented on all versions of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The authors have no fnancial or proprietary interests in any material discussed in this article.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dorna, D., Paluszczak, J. Targeting cancer stem cells as a strategy for reducing chemotherapy resistance in head and neck cancers. J Cancer Res Clin Oncol 149, 13417–13435 (2023). https://doi.org/10.1007/s00432-023-05136-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05136-9