Abstract
Background
Patients with clear cell renal cell carcinoma (ccRCC), which is the most commonly diagnosed subtype of renal cell carcinoma, are at risk of tumor metastasis and recrudescence. Previous research has shown that oxidative stress can induce tumorigenesis in many cancers and can be a target of cancer treatment. Despite these findings, little progress has been made understanding in the association of oxidative stress-related genes (OSRGs) with ccRCC.
Methods
In vitro experiments were conducted with MTT survival assays, qRT‒PCR, apoptosis assays, cell cycle assays, ROS assays, and IHC staining.
Results
In our study, 12 differentially expressed oxidative stress-related genes (DEOSGs) and related transcription factors (TFs) that are relevant to overall survival (OS) were screened, and their mutual regulatory networks were constructed with data from the TCGA database. Moreover, we constructed a risk model of these OSRGs and performed clinical prognostic analysis and validation. Next, we performed protein–protein interaction (PPI) network analysis and Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of MELK, PYCR1, and PML. A tissue microarray also verified the high expression of MELK and PYCR1 in ccRCC. Finally, in vitro cellular experiments demonstrated that knockdown of MELK or PYCR1 significantly inhibited ccRCC cell proliferation by causing cell apoptosis and inducing cell cycle arrest in the G1 phase. Intracellular ROS levels were elevated after these two genes were knocked down.
Conclusion
Our results revealed the potential DEORGs to be used in ccRCC prognostic prediction and identified two biomarkers, named PYCR1 and MELK, which regulated the proliferation of ccRCC cells by affecting ROS levels. Furthermore, PYCR1 and MELK could be promising targets for predicting the progression and prognosis of ccRCC, thereby serving as new targets for medical treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma, a tumor characterized by high incidence and mortality rates, poses a gradually increasing threat to human health (Siegel et al. 2017). ccRCC is the most common histological subtype of renal cell carcinoma, and it accounts for more than 80% of renal cell carcinoma cases (Barth et al. 2019). Recently, cancer genome studies have revealed the great intra- and intertumor heterogeneity of ccRCC, and its clinical symptoms and prognosis are even more elusive to physicians (Hsieh et al. 2017). For patients with early, localized cancer, the five-year survival rate can reach 90% after treatment with current therapeutic methods, including TKIs, cabozantinib, and immunotherapy. In contrast, five-year survival decreases to 12% for patients with distant metastatic disease (Atkins and Tannir 2018). Although the use of targeted molecular therapy in ccRCC patients has shown remarkable achievements, the clinical efficacy is not yet satisfactory for patients with tumor recurrence or metastasis (Yang and Chen 2020). Therefore, it is critical to explore more effective diagnostic markers and novel strategies to improve therapeutic effects in patients with advanced ccRCC.
Oxidative stress, which is a universal pathological phenomenon, basically refers to an excess of reactive oxygen species (ROS) relative to antioxidants (Hayes et al. 2020). In normal cells, when the antioxidant defense system is insufficient for coping with the damage caused by oxidative stress, continued exposure to high levels of ROS can damage intracellular components such as DNA and thus increase the risk of carcinoma (Rose et al. 2020; Gill et al. 2016). In contrast, in cancer cells, ROS affects many aspects of tumor progression, including cell proliferation, evasion of apoptosis or anoikis, invasion and metastasis, and angiogenesis (Sosa et al. 2013). Notably, oxidative stress in cells can lead to the disruption of the intracellular redox balance (Abu et al. 2017; Day et al. 2012). This disrupted balance contributes to the excessive accumulation of ROS, which can cause clear damage to the cellular structure, disrupting cell metabolism and destroying nucleic acid stability, thereby causing cell death (Prasad et al. 2017). Recent research has concluded that the induction of oxidative stress can preferentially kill cancer cells due to the higher sensitivity of cancer cells to further accumulation of ROS (Trachootham et al. 2009). Various drugs that direct target ROS metabolism are already available in clinical medicine, such as NOV-002, sulfasalazine and celecoxib (Trachootham et al. 2009). The level of ROS is significantly elevated in renal cancer patients, and previous research has shown that cellular redox homeostasis changes significantly in ccRCC (Ganesamoni et al. 2012; Pelicano et al. 2004). Previous research demonstrated that coadministration of 5-FU and belinostat increased ROS and DNA damage, and this combination can be used in the clinical treatment of metastatic renal cell carcinoma (Kim et al. 2015). Overall, substantial research has provided insights into changes in oxidative stress that play crucial roles in the progression of renal cell carcinoma. Oxidative stress has been proven to be a significant target in the treatment of cancer. However, little progress has been made in understanding the relationship of OSRGs with ccRCC. Accordingly, identifying potential novel OSRGs is the top priority for ccRCC research.
Notably, the changing characteristics of the tumor microenvironment (TME) are closely associated with the tumorigenesis, progression, and metastasis of tumors via various mechanisms. The TME consists of tumor cells and surrounding noncancerous components (including extracellular matrix, inflammatory cells, and immune cells) (Lai et al. 2021). There is increasing evidence that the components of the TME can cause oxidative stress in infiltrating immune cells, and low immunogenicity of tumor cells can impair the effect of anticancer drugs and play a significant role in tumor immune escape (Sheng et al. 2017). To cope with the decrease in oxygen levels in the TME, tumor cells generally undergo metabolic and oxidative stresses, which can restrict tumor growth but can also lead to an increase in the degree of malignancy by altering the tumor phenotype (Luis et al. 2021; Sanna and Rofstad 1994). In solid tumors, ROS are not only produced by tumor cells under oxidative stress but also can be produced by activated immune cells, such as neutrophils and macrophages (Meng et al. 2021). Increasing evidence suggests that elevated ROS levels in the TME could limit the killing ability of effector immune cells, which is strongly associated with the generation of an immunosuppressive tumor environment (Scortegagna et al. 2003). Therefore, in-depth exploration of the correlation between immune cell infiltration in the TME and oxidative stresses in ccRCC will be helpful for further identifying reliable molecular markers as well as novel strategies that target the tumor redox balance.
In this study, we screened 12 DEORGs and constructed a predictive risk model derived from the TCGA database. Then, we chose two key genes, PYCR1 and MELK, that may be potential oxidative stress-related biomarkers. Finally, we verified the transcription and protein expression levels of these two genes in clinical tumor tissues and conducted in vitro experiments to determine the role of PYCR1 and MELK in immune infiltration and OS in ccRCC, thereby determining the mechanism by which these genes affect tumor progression.
Methods and materials
Raw data and filtering
The transcription profiling data of 611 ccRCC samples (72 normal samples and 539 tumor samples) and clinical data were obtained from the TCGA database. The clinical data that contained invalid information were deleted. Ninety-seven OSRGs were acquired from the Gene Set Enrichment Analysis (GSEA) database. Transcription factors (TFs) were obtained from the Cistrome Cancer database (http://www.cistrome.org/).
Differential gene expression analysis
The “limma” package was used to analyze the expression of OSRGs. |log2-fold change (FC)| equal to or greater than 1 and an adjusted P value less than 0.05 were considered the thresholds for identifying statistically significant differentially expressed used (DEGs). The R packages “pheatmap” and “ggplot2” were applied to visualize the results.
Estimation of risk model
The “survival” package in R was used to perform univariate Cox regression analysis and multivariate Cox regression analysis. The survival analysis was performed via the R packages “survival” and “survminer”. Then, the survival curve was generated with the Kaplan–Meier method. The “survivalROC” and “timeROC” packages in R were used to graph the ROC curve. The nomogram model was plotted via the “rms” package in R.
Enrichment analysis
The STRING database (http://string-db.org/) was used for predicting a potential protein binding network. The GO and KEGG pathway enrichment analysis were performed through the R package “ggplot2” and“clusterProfiler”.
Cell culture and siRNA transfection
The ACHN cell line was cultured in MEM, and the Caki-1 cell line was cultured in McCoy’s 5A medium. Necessarily, all media were supplemented with 10% fetal bovine serum (FBS). The cell lines were obtained from the Cell Bank of the Chinese Academy of Science and were authenticated and tested for mycoplasma contamination before use. The siRNAs were obtained from GenePharma. The sequences of the siRNAs (PYCR1 and MELK) are as follows:siPYCR1: 5′-GCCACAGUUUCUGCUCUCATT-3′; antisense 5′-UGAGAGCAGAAACUGUGGCTT-3′. siMELK: 5′-CCUGGAUCAUGCAAGAUUATT-3′;antisense 5′-UAAUCUUGCAUGAUCCAGGTT-3′; siNC 5′-UUCUCCGAACGUGUCACGUTT-3′. Lipofectamine 3000 (Invitrogen, L3000015) was used to transfect cells when cell confluence reached 30%.
RNA extraction and qRT‒PCR
The RNA extraction assay was performed using an RNeasy mini kit (Qiagen) at 4 °C. Next, the RNA (1 μg) was reverse transcribed to cDNA. qRT‒PCR was then performed with SYBR Green PCR Master Mix according to the manufacturer's instructions. The relevant primer data sequences were as follows:
PYCR1: 5′-TGGCTGCCCACAAGATAATGG-3′; 5′-CGTGACGGCATCAATCAGGT-3′.
MELK: 5′-TCTCCCAGTAGCATTCTGCTT-3′;
5′-TGATCCAGGGATG GTTCAATAGA-3′.
GAPDH: 5′-GGAGCGAGATCCCTCCAAAAT-3′;
5′-GGCTGTTGTCATACTTCTCATGG-3′.
MTT assay
For the MTT assay, ACHN and Caki-1 cells were seeded in a 96-well plate at 1 × 103 cells per well and cultured overnight. After culturing for the indicated time, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg/ml) was added to each well and incubated for 4 h at 37 ℃. Then, the supernatants were discarded, and 200 µl of DMSO was added to each well. After shaking for 30 min, the absorbance at 540 nm was measured with a microplate reader (Bio-Rad, USA) to measure cell viability.
Analysis of ROS production, cell cycle, and apoptosis by flow cytometry
Cellular ROS levels were measured using flow cytometry (Beckman, USA). Briefly, ACHN and Caki-1 cells were incubated with 10 µM 2′,7′-dichlorodihydrofluorescein diacetate (Sigma‒Aldrich, USA) for 30 min at 37 °C. Next, the cells were washed three times with PBS and analyzed by flow cytometry. To analyze cell apoptosis, cells were stained with Annexin V FITC Apoptosis Assay Kit I (Sungene Biotech, China) according to the instructions, followed by flow cytometry. For the cell cycle assay, cells were stained with 1000 µl of 1 × DNA Staining Solution and 10 µl of permeabilization solution (Multi sciences, China).
Immunohistochemical (IHC) staining and tissue microarray
First, paraffin sections were incubated in citrate buffer to retrieve the antigens. Then, the sections were incubated in 3% H2O2. Then, they were incubated with the appropriate anti-MELK (Abclonal, A3530) or anti-PYCR1 (Abclonal, A13346) primary antibody followed by a secondary antibody (Anti-Rabbit-IgG H&L-HRP; Goat; Abcam, USA, cat. no. ab205718). Finally, DAB chromogen solution and HRP substrate solution were used to block the sections.
The ccRCC tissue and matched margin tissue combination tissue microarrays were purchased from Wuhan Baiqiandu Technology Co., Ltd. Specifically, the details of each sample tissue microarray are described in Supplementary Table 1. The MELK and PYCR1 protein levels were determined by calculating the percent of staining [i.e., 0 (0–5%), 1 (6–25%), 2 (26–50%), 3 (51–75%), and 4 (> 75%)] and the intensity of staining [i.e., 0 (negative), 1 (weak), 2 (moderate) and 3 (strong)] in each sample. A final immunoreactivity score (IRS) was obtained by adding the percentage and intensity scores.
Statistical analysis
All the results were obtained from more than three independent experiments. We performed survival analysis by means of the Kaplan–Meier estimate method and counted via the log-rank test. We used GraphPad Prism 7 (USA) statistical software and R software to analyze differences between groups by two-tailed t test. Statistical significance was displayed as follows: ns = not statistically significant, *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Identification of DEOSGs and construction of prognostic model
Volcano plots were generated to visualize the expression of DEOSGs in tumor tissues compared with normal tissues. The upregulated genes are indicated by red dots, while downregulated genes are represented by green dots (Fig. 1A). According to the DEG expression analysis, FZD1, GPR37, IL10, LRRK2, MELK, MET, NOL3, P4HB, PDK1, PML, PYCR1, RACK1, SOD2, and TLR6 were highly expressed in ccRCC neoplastic tissues compared with peritumoral tissues (P < 0.05, Fig. 1B). Moreover, univariate Cox regression analysis showed that CYP1B1, FZD1, LRRK2, MELK, NCOA7, NOL3, P4HB, PML, PYCR1, SLC7A11, SOD2, and TLR6 were related to prognosis and could be potential risk factors for ccRCC patients (P < 0.05, Fig. 1C).
Construction of the risk model of DEOSGs
We developed a prognostic risk model according to patient survival status to further identify potential biomarkers for ccRCC patients. Patients were classified into high-risk and low-risk groups based on the risk score (Fig. 2A). Patients in the high-risk group tended to have a shorter lifespan (Fig. 2B). The heatmap shows the differential expression levels of 6 DEOSGs between the high- and low-risk groups (Fig. 2C). The Kaplan‒Meier survival curve suggested that patients with a lower risk score had a better survival rate than high-risk patients (Fig. 2D). Then, we generated an ROC curve chart to confirm the prognostic accuracy of the constructed risk model. The area under the curve (AUC) of the risk model was 0.722, indicating that it has superior predictive accuracy (Fig. 2E). Finally, to more accurately predict patient outcomes, we also constructed a prognostic nomogram from the TCGA dataset to determine the 1-, 2-, and 3-year OS for ccRCC patients, which included diverse prognostic parameters, such as risk score, age, sex, and TNM staging (Fig. 2F). The calibration charts showed that the 3-year OS predictions were highly related to the actual observations (Fig. 2G).
Construction of risk model of DEOSGs. A, B Risk score distribution and survival status of TCGA ccRCC patient cohort. C Differential expression level of 6 DEOSGs between high- and low-risk groups in the heat map. D Kaplan-Meier survival of TCGA cohort. E The ROC curves for OS in TCGA cohort. F Nomogram of risk score and many clinical factors for predicting ccRCC 1-, 2-, and 3-year OS in TCGA cohort. G The calibration charts of the 3-year nomogram in TCGA cohort
Gene regulatory network of TFs and functional enrichment analysis of DEOSGs
To explore the potential regulatory mechanisms of transcription factors (TFs) and DEOSGs, we downloaded tumor-related TFs. Then, a total of 60 differentially expressed TFs were ultimately identified, including 41 upregulated TFs and 19 downregulated TFs (Fig. 3A, B). Moreover, we performed correlation analysis to predict the regulatory networks between these DEOSGs and differentially expressed TFs. Cytoscape software was used to visualize the TF-based regulatory networks. The construction of TF-based regulatory networks clearly revealed the regulatory relationships (Fig. 3C). Among the six DEOSGs, we focused on MELK, PYCR1, and PML, which have shown highly association with ccRCC patients’ outcomes. In order to decipher the underlying mechanisms by which the three key genes influenced tumorigenesis of ccRCC, we performed the PPI network analysis. As presented in Fig. 3D, the PYCR1-binding PPI networks, MELK-binding PPI networks and PML-binding PPI networks were created by the STRING online database. The GO enrichment analysis results revealed that the three key genes mainly correlated with immune response, immunoglobulin production and meiotic cell cycle. Moreover, the KEGG pathway analysis revealed 12 significantly enriched pathways, including cytokine-cytokine receptor interaction, cell cycle and neuroactive ligand-receptor interaction (Fig. 3E, F).
Gene regulatory network of TFs and functional enrichment analysis of DEOSGs. A, B Differentially TFs are shown in a volcano plot (A) and a heatmap (B). Green, red, and black dots represent genes expressed at relatively lower, higher, or equal levels. C A regulatory network comprising differentially expressed TFs and DEOSGs. Triangles represent TFs, and red and green indicate risk and protective factors, respectively. D The respective protein-protein interaction (PPI) network analysis of PML, PYCR1 and MELK from TCGA datasets. The colored dot indicates a straight functional protein cluster to the three key genes. (https://string-db.org/, http://gepia.cancer-pku.cn/index.html). E, F The GO and KEGG pathway enrichment analysis of PML, PYCR1 and MELK-related partners. Significantly enriched pathways are indicated in Y axis. Gene Ratio in the X axis represents the enrichment levels. The larger value of Gene Ratio represents the higher level of enrichment. KEGG, Kyoto encyclopedia of genes and genomes; GO, gene ontology
Clinical prognostic analysis and validation of hub gene expression
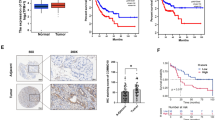
First, we analyzed the expression levels of MELK, PYCR1, and PML according to the TCGA database, and we showed that the three hub genes were upregulated in ccRCC tumor tissues compared with matched normal tissues (Fig. 4A). Based on the Kaplan–Meier Plotter database, we determined the association of MELK, PYCR1, and PML with OS. The OS curves indicated that patients with higher levels of the three genes had worse outcomes (Fig. 4B). Moreover, we measured the association between the mRNA expression of the three key genes and different clinical characteristics, which showed that their mRNA expression was positively related to tumor pathological grade, stage and TNM stage (Fig. 4C-E).
Clinical prognostic analysis and validation of hub genes. A Express levels of MELK, PYCR1, and PML in ccRCC tumor tissues or normal tissues in the TCGA database. B The overall survival curves of MELK, PYCR1, and PML according to the Kaplan–Meier Plotter database. C–E The association between the mRNA expression levels of MELK, PYCR1, PML and grade (C), stage (D), TNM stage (E)
Immune infiltration analysis of MELK, PYCR1 and PML
To determine the capability of the prognostic model to indicate the status of the tumor immune microenvironment, we conducted a systematic exploration of the association between hub genes and immune cell infiltration based on the TIMER database. As shown in Fig. 5A–C, MELK demonstrated a significant correlation with dendritic cells (cor = 0.37), neutrophils (cor = 0.34) and B cells (cor = 0.31). In addition, increased expression of PYCR1 and PML was significantly correlated with CD4+ T cell, neutrophil, and dendritic cell infiltration (P < 0.05). Moreover, we analyzed the underlying relationships between the mRNA levels of the three key genes and the immune status of ccRCC (P < 0.05). The results from the TISIDB database showed that MELK, PYCR1, and PML expression was obviously altered between diverse immune status of ccRCC, which suggested that the three key genes were highly correlated with immune infiltration in ccRCC (Fig. 5D).
Validation of elevated MELK and PYCR1 expression in ccRCC tissue by tissue microarray
To further determine whether MELK and PYCR1 expression was associated with clinical outcomes of ccRCC patients, we used a human ccRCC tissue microarray to perform an IHC assay. The specimens were collected from 38 ccRCC patients, including tumor tissues or adjacent tumor tissues, and stained for MELK (Fig. 6A). Moreover, the PYCR1 microarray contained 28 samples of ccRCC tissues and matched tumor edge renal tissues (Fig. 6B). As shown in Fig. 6E and 6F, the protein levels of MELK and PYCR1 were upregulated in tumor tissues compared with adjacent tissues. Additionally, the expression of MELK and PYCR1 was positively associated with the TNM stage of ccRCC patients (Fig. 6C, D).
MELK and PYCR1 expression were elevated in ccRCC tissue by the tissue microarray. A, B The expression of MELK and PYCR1 in ccRCC tissues and adjacent normal tissues was detected by TMAs. C, D Representative views showed the IHC staining of normal tissues and tumor tissues including different clinical pathological stages. The enlarged images of MELK were selected from Figure A (normal corresponded to A10; Tumor T1a corresponded to D05; Tumor T1b corresponded to E09; Tumor T2 corresponded to F09). The enlarged images of PYCR1 were selected from Figure B (normal corresponded to A09; Tumor T1 corresponded to B04; Tumor T2 corresponded to F06; Tumor T3 corresponded to C06). Scale bar is 50 μm. E, F IHC score of MELK and PYCR1 expression showed the up-regulated levels in ccRCC tissues than benign renal tissues
Knockdown of MELK and PYCR1 inhibited ccRCC cell proliferation by elevating ROS levels and inducing cell cycle arrest in the G1 phase and apoptosis
To verify the biological functions of MELK and PYCR1 in ccRCC cells, we knocked down the expression of MELK or PYCR1 in the Caki-1 and ACHN cell lines, respectively (Fig. 7A). The MTT assay results demonstrated that the knockdown of MELK or PYCR1 inhibited cell proliferation in both ccRCC cell lines (Fig. 7B). Apoptosis assays and cycle assays also verified that siMELK or siPYCR1 induced ccRCC cell apoptosis and cell cycle arrest in the G1 phase (Fig. 7C-D, Supplementary 7A-B). Moreover, the ROS levels were significantly increased after silencing the MELK or PYCR1 genes according to flow cytometry (Fig. 7E, Supplementary 7C).
Knockdown of MELK and PYCR1 inhibited ccRCC cell proliferation by elevating ROS level, inducing G1 phase cell cycle arrest and apoptosis. A The mRNA levels of MELK or PYCR1 was weakened by si-MELK and si-PYCR1 transfection in Caki-1 and ACHN cell lines. B Knockdown of MELK or PYCR1 inhibited the proliferation of the ACHN and Caki-1 cell lines. C, D The si-MELK or si-PYCR1 induced cell apoptosis increasing, cell G1 phase cycle arrest, and added intracellular ROS levels (E) in Caki-1 cell line
Discussion
ccRCC is one of the leading causes of malignant urinary tumors, and its incidence is increasing year by year (Siegel et al. 2018). Despite recent improvements in surgical therapy for early ccRCC, patients with high-stage tumors still suffer from high metastasis rates and poor prognoses (Patard et al. 2011; Fisher et al. 2013). Therefore, the exploration of novel tumor biomarkers for the early detection, prognostic assessment, and appropriate therapy of ccRCC is extremely urgent.
Recently, ccRCC has been classified as a “metabolic disease” due to common alterations in cellular metabolic pathways that are associated with cancer initiation and progression (Linehan et al. 2010). The enhanced metabolic activity of cancer cells results in ROS overproduction, leading to changes in many processes that are necessary for tumor initiation, growth, and progression (Sullivan and Chandel 2014). In light of ROS overproduction, ccRCC tumors express high levels of antioxidants, such as reduced glutathione (GSH), to prevent the accumulation of multiple ROS (Bansal et al. 2019). Furthermore, increased oxidative stress is an important factor that leads to abnormal intracellular signal transduction (Kim et al. 2019). ROS can induce continuous activation of the pSTAT3, NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways, regulate angiogenesis, accelerate tumor metastasis and promote the growth of ccRCC (Zhang et al. 2017). Due to the close association of ROS and their interactions with oxidative stress in ccRCC, it is necessary to research ROS-related biomarkers that can accurately predict clinical results and therapeutic targets.
In our research, 12 DEOSGs with prognostic value were screened, and an interaction network was constructed to identify the mechanism underlying the regulatory functions of these DEOSGs. Then, a multivariate Cox model was established to identify six prognosis-related hub genes, which were verified using statistical methods. The survival analysis revealed that the three key genes (MELK, PYCR1 and PML) could serve as independent prognostic factors. Furthermore, the results from the TIMER database elucidated that the mRNA expression of the three genes was strongly correlated with immune cell infiltration. These results demonstrated the prognostic value of a DEOSG-dependent risk model for patients with ccRCC. Moreover, the results indicated that these screened genes were significantly associated with ccRCC progression. However, previous studies have shown that PML plays crucial roles in the tumorigenesis of ccRCC (Lin et al. 2014). Therefore, we mainly focused on the search for PYCR1 and MELK and further confirmed that PYCR1 and MELK were critical for the comprehensive evaluation of the mechanism, which could be a novel direction for further research.
PYCR1 (pyrroline-5-carboxylate reductase 1), consisting of 10 exons, plays an important role in proline biosynthesis (Phang et al. 2015). Proline is a typical amino acid with secondary amines, and it is abundant in the cell microenvironment and is known as an indicator of various pathological stresses that occur during the process of tumorigenesis (Elia et al. 2017). However, as an active enzyme that catalyzes proline synthesis, PYCR might play significant roles in amino acid and energy metabolism, oxidative stress, and the malignant progression of some kinds of cancers (Chen et al. 2019). Under hypoxic conditions, the availability of proline is conducive to ATP generation or autophagy caused by ROS, which is essential for cancer cell growth and survival (Liu and Phang 2012). Due to these functions, proline may be associated with the abnormal expression of PYCR1 in cancer cells. Increasing evidence suggests that PYCR1 is highly expressed in various cancers, such as prostate cancer (Zeng et al. 2017), non-small cell lung cancer (NSCLC) (Cai et al. 2018) and hepatocellular cancer (Zhuang et al. 2019). For example, previous research demonstrated that silencing of PYCR1 inhibited proliferation, invasive migration capability, epithelial-mesenchymal transition, and metastatic abilities in hepatocellular carcinoma (HCC) (Guo et al. 2021). In addition, PYCR1 was also reported to be overexpressed in NSCLC and to promote the development of NSCLC by activating the p38 pathway (Wang et al. 2019). Consistent with previous studies on PYCR1 in other cancers, our findings predicted that PYCR1 was overexpressed in ccRCC tissues, which indicated a poor prognosis for ccRCC patients. In vitro cell function experiments further confirmed that knockdown of PYCR1 inhibited the proliferation of ccRCC cells. Additionally, flow cytometry showed that the silencing PYCR1 can increase ROS levels, as well as the proportion of cells that are arrested in the G1 phase. Furthermore, studies have shown that PYCR1 is closely associated with the TME, which has a distinct impact on tumorigenesis (Chen et al. 2021). ROS-related inflammatory cytokines, such as IL-13 and VEGF-A, can polarize macrophages toward an M2-like phenotype (Kuo et al. 2020). As the primary immunosuppressive cells in the TME, the M2-like phenotype of tumor-associated macrophages (TAMs) can give rise to a state of immunosuppression, which is conducive to the occurrence of tumors (Mantovani et al. 2002). In our study, we showed that PYCR1 was highly correlated with immune infiltration in ccRCC. Taken together, our data showed that PYCR1 may be a promising biomarker in ccRCC.
Maternal embryonic leucine zipper kinase (MELK) was first identified in Xenopus oocytes and embryos. It is a member of the AMP-activated protein kinase family of serine-threonine kinases (Thangaraj et al. 2020). In addition, the main posttranslational modification regulation of MELK relies on its 16 autophosphorylation sites (Seong et al. 2017). The inactivation of MELK is dependent on the direct phosphorylation of thioredoxin (Trx) at the Thr76 site (Manoharan et al. 2013). When MELK is activated by ASK1/TGF-β/p53 signals, Trx dissociates from MELK, and ZPR9 binds to the Thr252 site (Seong et al. 2002). As the focal point of several signal transduction pathways, MELK can regulate various bioprocesses, such as apoptosis, cell cycle arrest, and cell proliferation (Thangaraj et al. 2020). The earliest studies showed that MELK can directly phosphorylate and activate apoptosis signal-regulating kinase 1 (ASK1), which mediates the JNK and p38 signaling pathways and induces cell death (Jung et al. 2008; Seong et al. 2012). Moreover, MELK has been shown to regulate apoptosis and cell growth by affecting TGF-β-mediated signaling (Seong et al. 2010). Other studies have also shown that MELK can interact with p53 to regulate its function and affect normal cellular physiological functions (Seong and Ha 2012). Although MELK expression is tightly regulated in normal cells, it is significantly upregulated in a variety of tumor tissues (Thangaraj et al. 2020). Many studies have revealed that MELK performs tumor-promoting functions regarding the tissue origin of the cancer, and high expression of MELK is positively correlated with poor patient prognosis (Thangaraj et al. 2020; Lee et al. 2007; Martín-Martín et al. 2016). Recently, MELK has been defined as a crucial oncogene that plays indispensable roles in the Akt/mTOR signaling pathway to mediate the progression of colorectal cancer (CRC) (Tang et al. 2022). Moreover, MELK has been reported to be associated with the tumorigenesis of lung adenocarcinoma (LUAD) by increasing the migration and invasion of LUAD cells by regulating the EMT signal pathway. In recent years, an increasing number of potential therapeutic methods have focused on redox homeostasis in ccRCC, such as supplementation with antioxidants to target elevated ROS levels, which leads to the apoptosis of tumor cells (Mihailovic et al. 2021; Zacharias et al. 2021). Our current data also showed that downregulation of MELK could lead to an elevation in ROS generation. Whether MELK-related oxidative stress determines influences cellular homeostasis and the prognosis of ccRCC cells will require further investigation.
In the present study, we identified PYCR1 and MELK as prognosis-associated DEOSGs via a variety of bioinformatics analyses and verifications, and these genes were upregulated in human ccRCC clinical specimens in comparison to normal tissues. Moreover, in the TCGA database, the upregulation of PYCR1 and MELK was associated with late clinical features (grade, stage, TNM stage) and poor prognosis in ccRCC patients. In addition, based on the TIMER and TISIDB databases, we determined that PYCR1 and MELK were independently correlated with immune infiltration in ccRCC. This indicated that both genes may be important immune biomarkers and immunotherapy targets in ccRCC therapy. To verify the effects of PYCR1 and MELK on oxidative stress, we conducted an ROS assay to indicate that they play critical roles in the regulation of redox equilibrium. Inevitably, however, our study has some limitations. On the one hand, this study was based on open-source databases, and the clinical information was probably incomplete and limited. On the other hand, the specific mechanism underlying the change in expression of the two genes during oxidative stress in ccRCC still needs further study. We need to conduct further experiments to verify the specific biological mechanisms of PYCR1 and MELK as well as the tumor-immune axis in ccRCC. In conclusion, we generated a risk model comprising the ROS-related genes PYCR1 and MELK, which may contribute to regulating the development of ccRCC progression and serve as independent prognostic factors for patients with ccRCC.
Conclusions
In summary, our present study analyzed and identified two crucial DEOSGs (PYCR1 and MELK) using integrated bioinformatics analysis. Then, we demonstrated the overexpression of PYCR1 and MELK in ccRCC and verified this finding by analyzing tumor specimens, and overexpression of these genes indicates a poor prognosis for ccRCC patients. These two novel biomarkers could provide us with refreshing insights into the diagnosis, progression and risk prediction of ccRCC patients. Furthermore, these findings could lead to the development of promising personalized and precision therapies combined with these two biomarkers for ccRCC.
Data availability
The data that support the findings of this study are openly available in The Cancer Genome Atlas (TCGA) database at https://genomecancer.ucsc.edu/.
References
Abu AO, Habib SL, Trott J, Stewart B, Liang S, Chaudhari AJ, Sutcliffe J, Weiss RH (2017) Glutamine addiction in kidney cancer suppresses oxidative stress and can be exploited for real-time imaging. Cancer Res 77(23):6746–6758. https://doi.org/10.1158/0008-5472.CAN-17-0930
Atkins MB, Tannir NM (2018) Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev 70:127–137. https://doi.org/10.1016/j.ctrv.2018.07.009
Bansal A, Sanchez DJ, Nimgaonkar V, Sanchez D, Riscal R, Skuli N, Simon MC (2019) Gamma-glutamyltransferase 1 promotes clear cell renal cell carcinoma initiation and progression. Mol Cancer Res 17(9):1881–1892. https://doi.org/10.1158/1541-7786.MCR-18-1204
Barth DA, Slaby O, Klec C, Juracek J, Drula R, Calin GA, Pichler M (2019) Current concepts of non-coding rnas in the pathogenesis of non-clear cell renal cell carcinoma. Cancers (Basel). https://doi.org/10.3390/cancers11101580
Cai F, Miao Y, Liu C, Wu T, Shen S, Su X, Shi Y (2018) Pyrroline-5-carboxylate reductase 1 promotes proliferation and inhibits apoptosis in non-small cell lung cancer. Oncol Lett 15(1):731–740. https://doi.org/10.3892/ol.2017.7400
Chen S, Yang X, Yu M, Wang Z, Liu B, Liu M, Liu L, Ren M, Qi H, Zou J, Vucenik I, Zhu WG, Luo J (2019) SIRT3 regulates cancer cell proliferation through deacetylation of PYCR1 in proline metabolism. Neoplasia 21(7):665–675. https://doi.org/10.1016/j.neo.2019.04.008
Chen D, Zhang X, Li Z, Zhu B (2021) Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 11(3):1016–1030. https://doi.org/10.7150/thno.51777
Day AM, Brown JD, Taylor SR, Rand JD, Morgan BA, Veal EA (2012) Inactivation of a peroxiredoxin by hydrogen peroxide is critical for thioredoxin-mediated repair of oxidized proteins and cell survival. Mol Cell 45(3):398–408. https://doi.org/10.1016/j.molcel.2011.11.027
Elia I, Broekaert D, Christen S, Boon R, Radaelli E, Orth MF, Verfaillie C, Grünewald T, Fendt SM (2017) Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat Commun 8:15267. https://doi.org/10.1038/ncomms15267
Fisher R, Gore M, Larkin J (2013) Current and future systemic treatments for renal cell carcinoma. Semin Cancer Biol 23(1):38–45. https://doi.org/10.1016/j.semcancer.2012.06.004
Ganesamoni R, Bhattacharyya S, Kumar S, Chauhan A, Mete UK, Agarwal MM, Mavuduru R, Kaushik G, Mandal AK, Singh SK (2012) Status of oxidative stress in patients with renal cell carcinoma. J Urol 187(4):1172–1176. https://doi.org/10.1016/j.juro.2011.11.105
Gill JG, Piskounova E, Morrison SJ (2016) Cancer, oxidative stress, and metastasis. Cold Spring Harb Symp Quant Biol 81:163–175. https://doi.org/10.1101/sqb.2016.81.030791
Guo J, Cheng X, Tian Y, Li B, Zhang X, Gao X, An Y (2021) Knockdown of PYCR1 suppressed the malignant phenotype of human hepatocellular carcinoma cells via inhibiting the AKT pathway activation. Reprod Biol 21(3):100534. https://doi.org/10.1016/j.repbio.2021.100534
Hayes JD, Dinkova-Kostova AT, Tew KD (2020) Oxidative stress in cancer. Cancer Cell 38(2):167–197. https://doi.org/10.1016/j.ccell.2020.06.001
Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V (2017) Renal cell carcinoma. Nat Rev Dis Primers 3:17009. https://doi.org/10.1038/nrdp.2017.9
Jung H, Seong HA, Ha H (2008) Murine protein serine/threonine kinase 38 activates apoptosis signal-regulating kinase 1 via Thr 838 phosphorylation. J Biol Chem 283(50):34541–34553. https://doi.org/10.1074/jbc.M807219200
Kim MJ, Lee JS, Park SE, Yi HJ, Jeong IG, Kang JS, Yun J, Lee JY, Ro S, Lee JS, Choi EK, Hwang JJ, Kim CS (2015) Combination treatment of renal cell carcinoma with belinostat and 5-fluorouracil: a role for oxidative stress induced DNA damage and HSP90 regulated thymidine synthase. J Urol 193(5):1660–1668. https://doi.org/10.1016/j.juro.2014.11.091
Kim EK, Jang M, Song MJ, Kim D, Kim Y, Jang HH (2019) Redox-mediated mechanism of chemoresistance in cancer cells. Antioxidants (Basel). https://doi.org/10.3390/antiox8100471
Kuo CL, Chou HY, Chiu YC, Cheng AN, Fan CC, Chang YN, Chen CH, Jiang SS, Chen NJ, Lee AY (2020) Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett 474:138–150. https://doi.org/10.1016/j.canlet.2020.01.019
Lai Y, Tang F, Huang Y, He C, Chen C, Zhao J, Wu W, He Z (2021) The tumour microenvironment and metabolism in renal cell carcinoma targeted or immune therapy. J Cell Physiol 236(3):1616–1627. https://doi.org/10.1002/jcp.29969
Lee HE, Jee CD, Kim MA, Lee HS, Lee YM, Lee BL, Kim WH (2007) Loss of promyelocytic leukemia protein in human gastric cancers. Cancer Lett 247(1):103–109. https://doi.org/10.1016/j.canlet.2006.03.034
Lin YC, Lu LT, Chen HY, Duan X, Lin X, Feng XH, Tang MJ, Chen RH (2014) SCP phosphatases suppress renal cell carcinoma by stabilizing PML and inhibiting mTOR/HIF signaling. Cancer Res 74(23):6935–6946. https://doi.org/10.1158/0008-5472.CAN-14-1330
Linehan WM, Srinivasan R, Schmidt LS (2010) The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol 7(5):277–285. https://doi.org/10.1038/nrurol.2010.47
Liu W, Phang JM (2012) Proline dehydrogenase (oxidase), a mitochondrial tumor suppressor, and autophagy under the hypoxia microenvironment. Autophagy 8(9):1407–1409. https://doi.org/10.4161/auto.21152
Luis G, Godfroid A, Nishiumi S, Cimino J, Blacher S, Maquoi E, Wery C, Collignon A, Longuespée R, Montero-Ruiz L, Dassoul I, Maloujahmoum N, Pottier C, Mazzucchelli G, Depauw E, Bellahcène A, Yoshida M, Noel A, Sounni NE (2021) Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and fatty acid biding protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol 43:102006. https://doi.org/10.1016/j.redox.2021.102006
Manoharan R, Seong HA, Ha H (2013) Thioredoxin inhibits MPK38-induced ASK1, TGF-β, and p53 function in a phosphorylation-dependent manner. Free Radic Biol Med 63:313–324. https://doi.org/10.1016/j.freeradbiomed.2013.05.020
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555. https://doi.org/10.1016/s1471-4906(02)02302-5
Martín-Martín N, Piva M, Urosevic J, Aldaz P, Sutherland JD, Fernández-Ruiz S, Arreal L, Torrano V, Cortazar AR, Planet E, Guiu M, Radosevic-Robin N, Garcia S, Macías I, Salvador F, Domenici G, Rueda OM, Zabala-Letona A, Arruabarrena-Aristorena A, Zúñiga-García P, Caro-Maldonado A, Valcárcel-Jiménez L, Sánchez-Mosquera P, Varela-Rey M, Martínez-Chantar ML, Anguita J, Ibrahim YH, Scaltriti M, Lawrie CH, Aransay AM, Iovanna JL, Baselga J, Caldas C, Barrio R, Serra V, Vivanco M, Matheu A, Gomis RR, Carracedo A (2016) Stratification and therapeutic potential of PML in metastatic breast cancer. Nat Commun 7:12595. https://doi.org/10.1038/ncomms12595
Meng Y, Cai K, Zhao J, Huang K, Ma X, Song J, Liu Y (2021) Transcriptional profiling reveals kidney neutrophil heterogeneity in both healthy people and ccRCC patients. J Immunol Res 2021:5598627. https://doi.org/10.1155/2021/5598627
Mihailovic S, Coric V, Radic T, Radojevic AS, Matic M, Dragicevic D, Djokic M, Vasic V, Dzamic Z, Simic T, Hadzi-Djokic J, Pljesa EM (2021) The association of polymorphisms in Nrf2 and genes involved in redox homeostasis in the development and progression of clear cell renal cell carcinoma. Oxid Med Cell Longev 2021:6617969. https://doi.org/10.1155/2021/6617969
Patard JJ, Pignot G, Escudier B, Eisen T, Bex A, Sternberg C, Rini B, Roigas J, Choueiri T, Bukowski R, Motzer R, Kirkali Z, Mulders P, Bellmunt J (2011) ICUD-EAU international consultation on kidney cancer 2010: treatment of metastatic disease. Eur Urol 60(4):684–690. https://doi.org/10.1016/j.eururo.2011.06.017
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Update 7(2):97–110. https://doi.org/10.1016/j.drup.2004.01.004
Phang JM, Liu W, Hancock CN, Fischer JW (2015) Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care 18(1):71–77. https://doi.org/10.1097/MCO.0000000000000121
Prasad S, Gupta SC, Tyagi AK (2017) Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett 387:95–105. https://doi.org/10.1016/j.canlet.2016.03.042
Rose LY, Halliwill KD, Adams CJ, Iyer V, Riva L, Mamunur R, Jen KY, Del RR, Fredlund E, Hirst G, Alexandrov LB, Adams D, Balmain A (2020) Mutational signatures in tumours induced by high and low energy radiation in Trp53 deficient mice. Nat Commun 11(1):394. https://doi.org/10.1038/s41467-019-14261-4
Sanna K, Rofstad EK (1994) Hypoxia-induced resistance to doxorubicin and methotrexate in human melanoma cell lines in vitro. Int J Cancer 58(2):258–262. https://doi.org/10.1002/ijc.2910580219
Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA (2003) Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nat Genet 35(4):331–340. https://doi.org/10.1038/ng1266
Seong HA, Ha H (2012) Murine protein serine-threonine kinase 38 activates p53 function through Ser15 phosphorylation. J Biol Chem 287(25):20797–20810. https://doi.org/10.1074/jbc.M112.347757
Seong HA, Gil M, Kim KT, Kim SJ, Ha H (2002) Phosphorylation of a novel zinc-finger-like protein, ZPR9, by murine protein serine/threonine kinase 38 (MPK38). Biochem J 361(Pt 3):597–604. https://doi.org/10.1042/0264-6021:3610597
Seong HA, Jung H, Ha H (2010) Murine protein serine/threonine kinase 38 stimulates TGF-beta signaling in a kinase-dependent manner via direct phosphorylation of Smad proteins. J Biol Chem 285(40):30959–30970. https://doi.org/10.1074/jbc.M110.138370
Seong HA, Jung H, Manoharan R, Ha H (2012) PDK1 protein phosphorylation at Thr354 by murine protein serine-threonine kinase 38 contributes to negative regulation of PDK1 protein activity. J Biol Chem 287(25):20811–20822. https://doi.org/10.1074/jbc.M111.331827
Seong HA, Manoharan R, Ha H (2017) Zinc finger protein ZPR9 functions as an activator of AMPK-related serine/threonine kinase MPK38/MELK involved in ASK1/TGF-β/p53 signaling pathways. Sci Rep 7:42502. https://doi.org/10.1038/srep42502
Sheng X, Parmentier JH, Tucci J, Pei H, Cortez-Toledo O, Dieli-Conwright CM, Oberley MJ, Neely M, Orgel E, Louie SG, Mittelman SD (2017) Adipocytes sequester and metabolize the chemotherapeutic daunorubicin. Mol Cancer Res 15(12):1704–1713. https://doi.org/10.1158/1541-7786.MCR-17-0338
Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics. CA Cancer J Clin 67(1):7–30. https://doi.org/10.3322/caac.21387
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30. https://doi.org/10.3322/caac.21442
Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Lleonart ME (2013) Oxidative stress and cancer: an overview. Ageing Res Rev 12(1):376–390. https://doi.org/10.1016/j.arr.2012.10.004
Sullivan LB, Chandel NS (2014) Mitochondrial reactive oxygen species and cancer. Cancer Metab 2:17. https://doi.org/10.1186/2049-3002-2-17
Tang B, Zhu J, Liu F, Ding J, Wang Y, Fang S, Zheng L, Qiu R, Chen M, Shu G, Xu M, Lu C, Zhao Z, Yang Y, Ji J (2022) xCT contributes to colorectal cancer tumorigenesis through upregulation of the MELK oncogene and activation of the AKT/mTOR cascade. Cell Death Dis 13(4):373. https://doi.org/10.1038/s41419-022-04827-4
Thangaraj K, Ponnusamy L, Natarajan SR, Manoharan R (2020) MELK/MPK38 in cancer: from mechanistic aspects to therapeutic strategies. Drug Discov Today 25(12):2161–2173. https://doi.org/10.1016/j.drudis.2020.09.029
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8(7):579–591. https://doi.org/10.1038/nrd2803
Wang D, Wang L, Zhang Y, Yan Z, Liu L, Chen G (2019) PYCR1 promotes the progression of non-small-cell lung cancer under the negative regulation of miR-488. Biomed Pharmacother 111:588–595. https://doi.org/10.1016/j.biopha.2018.12.089
Yang DC, Chen CH (2020) Potential new therapeutic approaches for renal cell carcinoma. Semin Nephrol 40(1):86–97. https://doi.org/10.1016/j.semnephrol.2019.12.010
Zacharias NM, Wang L, Maity T, Li L, Millward SW, Karam JA, Wood CG, Navai N (2021) Prolyl hydroxylase 3 knockdown accelerates VHL-mutant kidney cancer growth in vivo. Int J Mol Sci. https://doi.org/10.3390/ijms22062849
Zeng T, Zhu L, Liao M, Zhuo W, Yang S, Wu W, Wang D (2017) Knockdown of PYCR1 inhibits cell proliferation and colony formation via cell cycle arrest and apoptosis in prostate cancer. Med Oncol 34(2):27. https://doi.org/10.1007/s12032-016-0870-5
Zhang Q, Yang Z, Han Q, Bai H, Wang Y, Yi X, Yi Z, Yang L, Jiang L, Song X, Kuang Y, Zhu Y (2017) G6PD promotes renal cell carcinoma proliferation through positive feedback regulation of p-STAT3. Oncotarget 8(65):109043–109060. https://doi.org/10.18632/oncotarget.22566
Zhuang J, Song Y, Ye Y, He S, Ma X, Zhang M, Ni J, Wang J, Xia W (2019) PYCR1 interference inhibits cell growth and survival via c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathway in hepatocellular cancer. J Transl Med 17(1):343. https://doi.org/10.1186/s12967-019-2091-0
Funding
This project was supported by grants from the program of Excellent Doctoral (Postdoctoral) of Zhongnan Hospital of Wuhan University (Grant No. ZNYB2019006 and ZNYB2021022), and Natural Science Foundation of Hubei Province (2021CFB055).
Author information
Authors and Affiliations
Contributions
DW conceived, designed the research. DW and ZD conducted the experiments, analyzed the data and wrote the manuscript. ML and KD participated in editing and refreshing the manuscript. FZ and ZL were responsible for experimental design and provided financial and instrumental support. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have declared that no competing interests exist.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki, and the informed consent from patients was granted by the Ethics Committee of Zhongnan Hospital of Wuhan University (No. 2020102).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
432_2023_4983_MOESM1_ESM.tif
Supplementary Figure 7. Knockdown of MELK and PYCR1 inhibited ccRCC cell proliferation by elevating ROS level, inducing G1 phase cell cycle arrest and apoptosis. (A) Knockdown of MELK or PYCR1 inhibited the proliferation of ACHN cell line. (B-C) The si-MELK or si-PYCR1 induced cell apoptosis increasing, cell G1 phase cycle arrest, and added intracellular ROS levels in ACHN cell line (TIF 17724 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Deng, Z., Lu, M. et al. Integrated analysis of the roles of oxidative stress related genes and prognostic value in clear cell renal cell carcinoma. J Cancer Res Clin Oncol 149, 11057–11071 (2023). https://doi.org/10.1007/s00432-023-04983-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-04983-w