Abstract
Purpose
HMGA2 has frequently been found in benign as well as malignant tumors and a significant association between HMGA2 overexpression and poor survival in different malignancies was described. In pancreatic ductal adenocarcinoma (PDAC), nuclear HMGA2 expression is associated with tumor dedifferentiation and presence of lymph node metastasis. Nevertheless, the impact of HMGA2 occurrence in other cell compartments is unknown.
Methods
Intracellular distribution of HMGA2 was analyzed in PDAC (n = 106) and peritumoral, non-malignant ducts (n = 28) by immunohistochemistry. Findings were correlated with clinico-pathological data. Additionally, intracellular HMGA2 presence was studied by Western blotting of cytoplasmic and nuclear fractions of cultured cells.
Results
HMGA2 was found in the cytoplasm and in the nucleus of cultured cells. In human tumor tissue, HMGA2 was also frequently found in the cytoplasm and the nucleus of tumor cells, however, nuclear staining was generally stronger. Direct comparison from tumor tissue with corresponding non-neoplastic peritumoral tissue revealed significantly stronger expression in tumors (p = 0.003). Of note, the nuclear staining was significantly stronger in lymph node metastatic cell nuclei compared to primary tumor cell nuclei (p = 0.049). Interestingly, cytoplasmic staining positively correlated with lymph vessel (p = 0.004) and venous invasion (p = 0.046).
Conclusion
HMGA2 is a prognostic marker in PDAC. Firstly, we found a positive correlation for cytoplasmic HMGA2 expression with lympho-vascular invasion and, secondly, we found a significantly stronger nuclear expression of HMGA2 in cancer-positive lymph node nuclei compared to primary tumor cell nuclei. So far, the role of cytoplasmic HMGA2 is nearly unknown, however, our data lend support to the hypothesis that cytoplasmic HMGA2 expression is involved in nodal spread.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Effective treatment of patients with pancreatic ductal adenocarcinoma (PDAC) requires early diagnosis and intervention. Although considerable efforts have been made to identify underlying molecular mechanism and novel sensitive specific tumor biomarkers, PDAC still remains one of the deadliest cancers with a mortality rate almost equal to its incidence rate (Siegel et al. 2020). Identification of reliable and reproducible biomarkers would enable better stratification of patients, and eventually provide a guide for individualized therapy. The high mobility group A2 (HMGA2/HMGI-C) is an architectural transcription factor and belongs to the high mobility group AT-hook (HMGA) gene family. It is highly expressed in embryonic tissue, whereas its expression drops during the differentiation being hardly detectable in healthy adult tissue (Chiappetta et al. 1996; Huang et al. 2018). Interestingly, HMGA2 is re-expressed and becomes again highly elevated in benign (Dreux et al. 2010; Tallini et al. 2000) as well as malignant neoplasms such as ovarian cancer (Wu and Wei 2013; Xi et al. 2014; Jin et al. 2018), breast cancer (Wu et al. 2016; Sgarra et al. 2018), lung cancer (Kumar et al. 2014), gastrointestinal cancer (Mito et al. 2017; Zhu et al. 2017; Huang et al. 2018; Wang et al. 2011; Zhang et al. 2016), and pancreatic cancer (Strell et al. 2017; Piscuoglio et al. 2012). Importantly, diverse meta-analyses revealed a correlation of high HMGA2 expression with poor patient’s survival in various malignancies such as gastric, colorectal as well as head-and-neck cancers (Binabaj et al. 2019; Nie et al. 2018; Huang et al. 2018). For hepatobiliary cancers, in particular HCC, cholangiocarcinoma and gallbladder cancer (Binabaj et al. 2019; Nie et al. 2018; Huang et al. 2018) as well as PDAC (Huang et al. 2018; Binabaj et al. 2019), poor survival was reported. Of note, not all tumors with elevated HMGA2 expression show significant association with survival rates (e.g., ovarian cancer Huang et al. 2018; Nie et al. 2018) or esophageal cancer (Huang et al. 2018). Thus, HMGA2 represents a reliable marker of prognostic value in some, but not all cancers.
Analyses of the HMGA2 expression in normal pancreatic tissue and pancreatic cancer revealed clearly elevated levels in the latter with significant association with malignancy (Hristov et al. 2009; Piscuoglio et al. 2012; Strell et al. 2017; Li et al. 2020). An increasing HMGA2 expression along with the PDAC development from normal pancreatic tissue, intraepithelial neoplasms (PanIN), and PDAC was detected (Piscuoglio et al. 2012; Strell et al. 2017) suggesting again its malignancy enhancing potential. In accordance, HMGA2 expression showed a positive correlation with tumor grade and progression: expression of HMGA2 increases upon dedifferentiation tumors (Hristov et al. 2009; Piscuoglio et al. 2012; Strell et al. 2017; Gong et al. 2019) and with the presence of lymph node metastases (Hristov et al. 2009; Piscuoglio et al. 2012; Gong et al. 2019; Li et al. 2020). Within recent studies, overall survival was found significantly associated with the level of HMGA2 expression (Haselmann et al. 2014; Strell et al. 2017; Gong et al. 2019; Li et al. 2020).
One of the known mechanisms underlying the pro-tumoral functions of HMGA2 is its role in the induction of epithelial–mesenchymal transition (EMT), a process linked to the acquisition of metastatic capability of tumor cells. Here, acting in its canonical way as a transcriptional regulator, HMGA2 enhances the expression of EMT regulators like Snail, Twist, Slug and ZEB1 thereby down regulating the levels of E-cadherin and upregulating vimentin (Thuault et al. 2008; Sgarra et al. 2018). Interestingly, Morishita et al. reported that overexpression of HMGA2 converted nonmetastatic 4TO7 breast cancer cells to metastatic cells that homed specifically to the liver in a mouse allograft model (Morishita et al. 2013). Of note, expression of HMGA2 is known to enhance different signaling pathways such as TGFβ signaling (Kou et al. 2018) which has been linked to metastasis (Xie et al. 2018). In addition, HMGA2 can induce EMT via MAPK (Watanabe et al. 2009; Hawsawi et al. 2018) and via the Wnt/β-catenin pathway (Zha et al. 2013). In accordance, HMGA2 smoothens the way for a metastatic phenotype and EMT in renal cell carcinoma (Kou et al. 2018), PDAC (Watanabe et al. 2009; Gong et al. 2019) and gastric cancer (Zha et al. 2013). Altogether, these and other data disclosed the role of HMGA2 as a key regulator of EMT and one of the major players in establishing a malignant phenotype in different tumors of epithelial origin, including pancreatic cancer.
Importantly, in the vast majority of histochemical studies analyzing the relevance of HMGA2 for cancer development, progression and disease outcome, a solely nuclear presence of this protein was analyzed. However, our recent report on HMGA2 in breast cancer clearly revealed a prognostic significance of cytoplasmic HMGA2. In particular, high levels of cytoplasmatic HMGA2 were associated with a favorable overall survival of breast cancer patients (Heilmann et al. 2020). In detail, HMGA2 expression was linked to better survival in triple negative breast cancer and well-differentiated estrogen receptor-positive breast cancer patients irrespective of lymph node metastases or tumor size. To the best of our knowledge, no comparable data are available so far for PDAC. To fill this gap of information, we tested in the present study the hypothesis that cytoplasmic expression of HMGA2 also impacts the malignant phenotype of PDAC.
Methods
Cell culture
The pancreatic cancer cell lines Panc1, Panc89, BxPC3 and colon carcinoma cell line HCT116 were cultured in RPMI 1640 supplemented with 10% FCS, 2 mM glutamine and 1 mM sodium pyruvate (all from Life Technologies Inc., Karlsruhe, Germany). For the preparation of nuclear and cytoplasmic cell extracts cells were grown for 24 h in 6-well plates and the NE-PER™ nuclear and cytoplasmic extraction reagents (Thermo Fischer Scientific, Darmstadt, Germany) were used according to the manufacturer’s protocol.
Western blot analysis
Nuclear and cytoplasmic cell fractions were separated on 4–20% Tris–Glycine gels (Invitrogen, Thermo Fisher Scientific, USA), blotted on PVDF-membrane and incubated with the appropriate primary antibody followed by incubation with the HRP-conjugated secondary antibody (Cell Signaling, Frankfurt, Germany). Antigen visualization was performed by enhanced chemiluminescence (ECL-kit, Amersham Pharmacia Biotech, England). Primary antibodies against HMGA2, α-tubulin, and lamin A/C (all rabbit) were purchased from Cell Signaling (Frankfurt, Germany).
Study cohort
For this study, formalin-fixed and paraffin-embedded PDAC and adjacent, peritumoral non-malignant tissue samples were used. Probes were retrieved form the archive of the Dept. of Pathology of the University Hospital Schleswig-Holstein and Christian-Albrechts-University Kiel spanning the period from 1999 to 2010. Follow-up data were obtained from the Epidemiological Cancer Registry Schleswig-Holstein, Germany and hospital records. Only patients with an adenocarcinoma of the pancreas were included. pTNM category was determined according to the 8th edition of the Union for International Cancer Control (UICC) guidelines (Brierley et al. 2016). Approval for this study was granted by the local institutional review board of the Medical Faculty of the Christian-Albrechts-University of Kiel (A-110/99).
Immunohistochemistry
Serial 3 µm paraffin sections were deparaffinized and rehydrated with xylene and rehydrated in a descending alcohol series. Antigen retrieval was done with citrate-buffer (pH 6.0) for 15 min at 120 °C, followed by blocking of endogenous peroxidase-activity with Hydrogen-Peroxide Block [15 min, room temperature (RT); Thermo Scientific, Fremont, CA]. Slides were incubated with primary antibody antibody (HMGI-C S-15) 1:50 (20 µg/ml) (Santa Cruz Biotechnology, Dallas, Texas) 1:50 (20 µg/ml) diluted in antibody diluent for 2 h at RT. Bound antibodies were visualized with the Histofine polymer (Histofine Simple Stain MAX PO Immuno-peroxidase Polymer Anti-Goat, Nichirei Biosciences, Tokyo, Japan) and diaminobenzidine (DAB Peroxidase Substrate Kit, Vector Laboratories, Burlingham, California). All slides were counterstained with hemalum and cover slipped.
Histopathological scoring
For evaluation of the staining, a two-dimensional scoring system was applied to semi-quantitatively assess the HMGA2 expression data on a Leica DM 1000-Microscope (Leica, Wetzlar, Germany) as described earlier (Gundlach et al. 2018). The intensity of the staining was defined on an arbitrary scale of 0–3 with 0: no staining; 1: weak staining; 2: moderate staining and 3: strong staining. In case of varying staining intensities, strongest values were recorded. Additionally, the percentage of stained cells was quantified and scaled from 0 to 4 with 0: no positive cells; 1: 1–10%; 2: 10–50%; 3: 51–80%; and 4: 81–100% positively stained cells. After being separately assessed for cytoplasm and nuclei by two independent pathologists, the values were summarized in a sum score as follows (Table 1): the addition of intensity and quantity scoring resulted in an immunoreactivity sum score. The sum score ranged from 0 to 7 for nuclear and cytoplasmatic immunoreactivity, respectively.
Statistical analyses
Statistical analyses were performed with SPSS 25.0 (SPSS, IBM Corporation, Armonk, NY, USA). Correlation of clinico-pathological patient characteristics and HMGA2 expression was conducted by dichotomization and appliance of Kendall’s Tau (τ) test. We included only patients with existing follow-up data, whereas patients who died within 14 days after surgery as well as patients who received neoadjuvant treatment were excluded. For these analyses 97 out of 106 patients were included, whereof, 19 patients were censored because they were either alive or lost in follow-up. We analyzed the overall postoperative survival. Evaluation of normal and malignant tissue staining intensities was performed with the Wilcoxon test as a nonparametric test for paired samples. Survival analyses were performed by Kaplan–Meier estimates with subsequent statistical evaluation by log-rank tests. p values ≤ 0.05 were considered significant.
Results
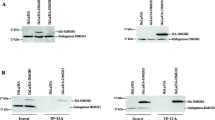
HMGA2 is localized to the cytosol and nucleus in different tumor cell lines
In the present study, we aimed to investigate whether the expression of HMGA2 and, in particular, the pattern of its intracellular distribution correlates with histopathological parameters and correlates with patient prognosis. To exclude the possibility of false-positive cytoplasmic immunostaining, we additionally performed Western blot analyses of cytosolic and nuclear extracts of three PDAC cell lines, i.e., Panc1, Panc89 and BxPC3, as well as the colon carcinoma cells HCT116. The results shown in Fig. 1 confirmed the presence of HMGA2 in the nuclei as well as in the cytoplasmic compartments in all four cell lines. In particular, for Panc1 and Panc89 cell lines, comparable expression was detected in cytosolic and nuclear fractions, while HMGA2 was more present in the nuclear fraction of BxPC3 cells and in the colon cancer cell line HCT116.
Patient cohort
In order to evaluate the staining intensity, percentage of stained cells and intracellular distribution of HMGA2 in sections of 106 tumors and 28 neighboring histological normal pancreatic ducts from 106 PDAC patients were analyzed as described before (Gundlach et al. 2018). Thereof, 51 (48.1%) patients were female and the median age of the whole cohort was 65 years (range 47–85 years). The anatomical location of the tumor was in the pancreatic head in 75/106 (70.8%), in the corpus in 7/106 (6.6%), and in the tail in 8/106 (7.5%) of the cases. No specification was stated in 16/106 cases (15.1%). We provide detailed clinico-pathological patient characteristics in Table 2. Almost 90 percent of the patients have undergone surgery at category T3 (94/106; 88.7%) with existing lymph node metastases (84/106; 79.2%). No patient was operated at category T1. Resected tumors were well or moderately differentiated in two-thirds (66.1%) of the cases. Distant metastasis was only present in 10.4% of the cases (11/106).
Expression of HMGA2 in PDAC and non-malignant adjacent tissue
HMGA2 was frequently found in tumor cells (Table 3). Representative images showing expression pattern of HMGA2 in tumor tissue and non-malignant, adjacent tissue are displayed in Fig. 2. In 86.8% (92/106), positive cytoplasmic staining and in 98.1% (104/106), positive nuclear staining was detected. Figure 2A + B represents tumors with nuclear staining without (A) and with simultaneous cytoplasm staining (B). In 76.4% (81/106) and 79.2% (84/106) of the cases, more than every second nucleus and every second cytoplasm was positively stained, respectively. Interestingly, the nuclear staining was in general stronger than the cytoplasmic staining (58.5% (62/106) moderate or strong in the nucleus vs. 3.8% (4/106) moderate or strong in the cytoplasm).
Representative images of HMGA2 staining in PDAC tissue (A, B), non-neoplastic pancreatic duct (C) and lymph node metastasis (D). A Tumors with strong nuclear staining in > 80% cells without cytoplasm staining; B tumors with cytoplasm and nuclear staining; C non-neoplastic duct with weak to moderate positive cytoplasm staining and with moderate to strong nuclei staining; D lymph node metastasis with strong nuclear staining. Scale bar marks 50 µm (A, B, D) as well as 100 µm (C)
HMGA2 was more frequently found both in the cytoplasm and the nuclei of tumor cells than in normal duct cells [Fig. 2C; cytoplasm 23/28 (82.1%) vs. 20/28 (71.4%) and nuclei 27/28 (96.4%) vs. 23/28 (82.1%)]. Moreover, cytoplasmic staining of HMGA2 was present with similarly low intensity in tumor and normal tissue (negative to weak positive in 28/28 and 26/28 cases, respectively; p = 0.5). In contrast, significant differences were found in the intensity of nuclear staining (negative to weak positive in 14/28 and 19/28 of the cases, respectively; p = 0.003), thereof, in 7 cases strong intensity was found in the nuclei of tumor cells. However, we could not detect any kind of mutual exclusion between staining of malignant and non-malignant tissue.
Expression of HMGA2 in lymph node metastases
In 17 patients, lymph node metastases were analyzed (Fig. 2D). HMGA2 was expressed in 64.7% (11/17) of the cytoplasm and in 94.1% (16/17) of the nuclei. Interestingly, HMGA2 staining was significantly stronger in lymph node nuclei compared to the individual corresponding nuclei of the primary tumor (p = 0.049). In contrast, the cytoplasmic presence of HGMA2 was not significantly different, neither in the staining intensity nor in the number of positive cells between the primary tumor and its lymph node metastases.
Correlation of HMGA2 expression with clinico-pathological parameters and patient survival
Subsequently, we correlated the expression level of HMGA2 and its intracellular distribution (cytoplasm and nucleus) with diverse clinico-pathological parameters (Table 4). A significant positive correlation of the nuclear HMGA2 staining intensity with tumor grading (τ = 0.193; p = 0.028) was assessed as previously described (Haselmann et al. 2014). Interestingly, the staining intensity of cytoplasmic HMGA2 positively correlated with lymph vessel invasion (p = 0.004) and the number of positively stained cells is associated with venous invasion (p = 0.046).
Moreover, we explored whether the HMGA2 expression pattern could be of prognostic value as shown in Table 5. To address this issue, we dichotomized the results for intensity and number of positive cells as well as the sum score in a group with strong and in a group with weak expression of HMGA2 and analyzed these data by Kaplan–Meier analysis (Fig. 3). Cumulative survival was compared by log-rank test and p values ≤ 0.05 were considered significant. Patients with a strongly positive expression for nuclear HMGA2 showed a significantly reduced overall survival (8 months vs. 16 months; p = 0.045) (Fig. 3B). However, neither the number of cells with positively stained nuclei nor their sum score showed a significant correlation (Fig. 3D + F). Furthermore, for cytoplasmic HMGA2 staining, no significant correlation with patient`s survival was detected. In detail, neither the intensity nor the number of positively stained cells nor the sum score showed a positive correlation (Fig. 3A, C + E).
Kaplan–Meier analyses of the cumulative survival of patients with differential expression of HMGA2. A, B HMGA2 intensity in the cytoplasm (A) and the nuclei (B). In the cytoplasm, no HMGA2 expression was dichotomized with weak to strong expression. In the nuclei, we dichotomized non to weak expression with moderate to strong expression. Graph C, D display patient survival in correlation with number of positively stained cells for the cytoplasm (C) and the nuclei (D), respectively. We compared equal to less than 80% positively stained cells with more than 80% positively stained cells with cytoplasm and nuclear staining, respectively. E, F Display survival curves for sum score with 0–4 points compared to > 4 points. p-values were calculated by the log-rank test and p ≤ 0.05 was considered significant
Discussion
HMGA2 was attributed as a prognostic marker in PDAC and different cancer types. Its expression is associated with advanced tumor grades, tumor dedifferentiation, lymph node metastases and poor patient prognosis (Hristov et al. 2009; Piscuoglio et al. 2012; Haselmann et al. 2014; Strell et al. 2017; Gong et al. 2019; Li et al. 2020). In the present study, we validated HMGA2 as a prognostic marker which correlates with malignant cell state in PDAC. We found a higher HMGA2 expression in tumor tissue compared with peritumoral tissue. Moreover, nuclear expression of HMGA2 was significantly stronger in lymph node nuclei than primary tumor cell nuclei. Correlation of HMGA2 expression with clinico-pathological parameters revealed a significant correlation of HMGA2 nuclei staining intensity with tumor grading. Moreover, a strong positive HMGA2 nuclei staining was associated with reduced overall survival. Importantly, along with nuclear expression of HMGA2 we also detected distinct cytoplasmic localization of HMGA2 in tumor cells. Cytoplasmic HMGA2 expression was found to positively correlate with lympho-vascular invasion.
Nuclear occurrence of HMGA2 has been known for a long time as it provides several nuclear functions including an involvement in cell cycle process, DNA damage repair, EMT, apoptosis, senescence and telomere restoration. HMGA2 is highly expressed in embryonic tissue whereas its expression is strictly downregulated in adult somatic cells (Chiappetta et al. 1996; Huang et al. 2018). Overexpression of HMGA2 has been attributed to a feature of malignancy. Nevertheless, HMGA2 could be detected in some non-malignant pancreatic ducts but its expression was higher in tumor tissue compared to non-malignant tissue, especially in the nuclei. This characteristic has been described before in PDAC (Abe et al. 2003; Hristov et al. 2009; Piscuoglio et al. 2012; Haselmann et al. 2014; Strell et al. 2017; Gong et al. 2019; Li et al. 2020). Apart from an expression in duct epithelia, we saw a subtle HMGA2 expression in acinar and endocrine cells with no difference in terms of expression pattern or color intensity. For this observation we do not have an explanation so far. This should be clarified in the context of further investigations.
HMGA2 is increasingly expressed in poorly differentiated tumors. In line with other studies, here we report a significant correlation of nuclei HMGA2 staining with tumor grading (Hristov et al. 2009; Piscuoglio et al. 2012; Gong et al. 2019; Strell et al. 2017; Li et al. 2020).
In addition to primary tumors and peritumoral non-malignant tissue, some lymph node metastases were also examined immunohistochemically. The intensity of nuclear staining was significantly higher in lymph node metastases compared to corresponding primary tumors. The number of patients from whom lymph node metastases could be examined was rather low with 17 patients. Hristov et al. demonstrated in a larger cohort a significant positive correlation of HMGA2 expression with lymph node metastases (Hristov et al. 2009). A high tumor grade and lymph node metastases are clinico-pathological parameters that are accompanied with worse patient prognosis. Correspondingly, patients with strong positive nuclear HMGA2 expression showed a significantly reduced overall survival (p = 0.035) (Strell et al. 2017; Haselmann et al. 2014; Gong et al. 2019).
The role of HMGA2 in EMT and metastatic spread has not yet been fully understood in pancreatic cancer (Gong et al. 2019), although, repeatedly studies have shown that overexpression of HMGA2 is accompanied by a more mesenchymal phenotype in several cancer cells. HMGA2 was described to be responsible in conjunction with the oncogenic RAS signaling pathway for cell growth and EMT in human pancreatic cancer cells (Watanabe et al. 2009). RAS and HMGA2 are known to be translationally downregulated by the let-7 microRNA family, and loss of let-7 expression led to progression of some human cancers (Johnson et al. 2005). Noteworthy, investigations by our group found a connection between TRAIL-R2 and let-7 microRNA. TRAIL-R2 was demonstrated to be located in the nucleus inhibiting the maturation of let-7 microRNA, leading to increased expression of HMGA2 in PDAC cells (Haselmann et al. 2014). In addition, staining intensities of nuclear HMGA2 and TRAIL-R2 showed a significant positive correlation (Haselmann et al. 2014).
In contrast to nuclear HMGA2, cytoplasmic HMGA2 has not been well characterized so far. In our dataset, we encouraged the histochemical cytoplasmic HMGA2 staining by analyzing cytoplasmic and nuclear fractions of three PDAC cell lines as well as a colon cancer cell line. Western blot results revealed an unmistakable detectability of HMGA2 in cytoplasmic cell lysates. Cytoplasmic HMGA2 has been also found by others in various tumor entities (Abe et al. 2003; Rahman et al. 2009; Gong et al. 2019; Heilmann et al. 2020). Noteworthy, yet, to the best of our knowledge, there are no reports analyzing the clinical relevance of the intracellular distribution of HMGA2 in PDAC. Unexpectedly, we found a correlation of cytoplasmic, but not of nuclear HMGA2 staining to lymphatic invasion and venous invasion in PDAC. This suggests that HMGA2 possesses distinct, compartment-dependent pro-tumoral functions.
Heilmann et al. described cytoplasmic HMGA2 as an autonomous phenomenon with a prognostic effect in breast cancer patients. High levels of cytoplasmic HMGA2 were associated with a favorable overall survival of breast cancer patients (Heilmann et al. 2020). However, the mechanism of how cytoplasmic HMGA2 favors patient survival needs further investigations. Although the extranuclear localization has long been recognized, little is known about possible functions of HMGA family proteins in this localization.
HMGA1 was described to translocate during late S- and G2-phase from the nucleus to the mitochondria (Nissen et al. 1991; Reeves et al. 1991; Dement et al. 2005). This movement is reported to be very dynamic, bidirectional and cell-cycle dependent. Furthermore, post-translational phosphorylation of HMGA1 proteins by cdc2 kinase alters the binding capacity of HMGA1 for DNA (Reeves et al. 1991) and favors its translocation. Nevertheless, in the mitochondria HMGA1 can bind to mitochondrial DNA (mtDNA) at the D-loop control region (Dement et al. 2005) and through this impacts on mitochondrial DNA maintenance and organelle functions (Dement et al. 2007). This transporting from the nucleus to the cytoplasm is deregulated in cancer cells (Dement et al. 2005). Interestingly, in malignant cells, HMGA1 is reported to be present in the cytoplasm throughout all stages of the cell cycle (Dement et al. 2007). In addition to mtDNA binding, here, HMGA1 inhibits p53-mediated apoptosis by blocking the binding of p53 to the anti-apoptotic factor Bcl-2 (Esposito et al. 2012). In addition to HMGA1, high mobility box 1 (HMGB1) proteins were also described in an extranuclear localization in the cytosol and mitochondria. Along with the receptor for advanced glycation end products, HMGB1 was described to enhance mitochondrial ATP production in malignant cells contributing to tumor progression (Kang et al. 2013). Nevertheless, cytoplasmic functions of HMGA2 have not been unraveled till now.
The prognostic impact especially of parameters like venous and lymphatic invasion is impeded by the overall bad prognosis and high lethality (due to other characteristics) of PDAC. Consequently, a possible prognostic impact of cytoplasmic HMGA2 staining intensity should be analyzed in other tumor entities with a better prognosis (for instance, early stage colorectal carcinoma) to fully evaluate the value of HMGA2 staining as a biomarker for better stratification of cancer patients, e.g., for adjuvant therapy.
Conclusion
In summary, our data indicate that HMGA2 might possess distinct, compartment-dependent pro-tumoral functions in PDAC. Not only nuclear, but also cytoplasmic expression of HMGA2 indicates a malignant phenotype. Interestingly, cytoplasmic HMGA2 significantly correlated with lymphatic and venous invasion. Thus, our data point to the necessity to investigate the unknown biological functions of cytoplasmic HMGA2.
Data availability
The clinical datasets supporting the conclusions of this study were derived from the patient files (paper and electronic form). Therefore, restrictions to availability apply due to data protection regulations. Anonymized data are, however, available from the corresponding author on reasonable request and with permission of the University Hospital Schleswig-Holstein and the local review board.
Abbreviations
- CI:
-
Confidence interval
- EMT:
-
Epithelial–mesenchymal transition
- G:
-
Grading
- HMGA2:
-
High mobility group A2
- IHC:
-
Immunohistochemistry
- L:
-
Lymphatic invasion
- M:
-
Distant metastasis
- N:
-
Nodal spread
- PanIN:
-
Pancreatic intraepithelial neoplasm
- PDAC:
-
Pancreatic ductal adenocarcinoma
- RT:
-
Room temperature
- SD:
-
Standard deviation
- T:
-
Tumor category
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- UICC:
-
Union for International Cancer Control
- V:
-
Venous invasion
References
Abe N, Watanabe T, Suzuki Y, Matsumoto N, Masaki T, Mori T, Sugiyama M, Chiappetta G, Fusco A, Atomi Y (2003) An increased high-mobility group A2 expression level is associated with malignant phenotype in pancreatic exocrine tissue. Br J Cancer 89:2104–2109
Binabaj MM, Soleimani A, Rahmani F, Avan A, Khazaei M, Fiuji H, Soleimanpour S, Ryzhikov M, Ferns GA, Bahrami A, Hassanian SM (2019) Prognostic value of high mobility group protein A2 (HMGA2) over-expression in cancer progression. Gene 706:131–139
Brierley JD, Gospodarowicz MK, Wittekind C (eds) (2016) TNM classification of malignant tumours, 8th edn. Wiley Blackwell
Chiappetta G, Avantaggiato V, Visconti R, Fedele M, Battista S, Trapasso F, Merciai BM, Fidanza V, Giancotti V, Santoro M, Simeone A, Fusco A (1996) High level expression of the HMGI (Y) gene during embryonic development. Oncogene 13:2439–2446
Dement GA, Treff NR, Magnuson NS, Franceschi V, Reeves R (2005) Dynamic mitochondrial localization of nuclear transcription factor HMGA1. Exp Cell Res 307:388–401
Dement GA, Maloney SC, Reeves R (2007) Nuclear HMGA1 nonhistone chromatin proteins directly influence mitochondrial transcription, maintenance, and function. Exp Cell Res 313:77–87
Dreux N, Marty M, Chibon F, Velasco V, Hostein I, Ranchere-Vince D, Terrier P, Coindre JM (2010) Value and limitation of immunohistochemical expression of HMGA2 in mesenchymal tumors: about a series of 1052 cases. Mod Pathol 23:1657–1666
Esposito F, Tornincasa M, Federico A, Chiappetta G, Pierantoni GM, Fusco A (2012) High-mobility group A1 protein inhibits p53-mediated intrinsic apoptosis by interacting with Bcl-2 at mitochondria. Cell Death Dis 3:e383
Gong J, Wang Y, Jiang B, Xu B, Hu C (2019) Impact of high-mobility-group A2 overexpression on epithelial–mesenchymal transition in pancreatic cancer. Cancer Manag Res 11:4075–4084
Gundlach JP, Hauser C, Schlegel FM, Böger C, Röder C, Röcken C, Becker T, Egberts JH, Kalthoff H, Trauzold A (2018) Cytoplasmic TRAIL-R1 is a positive prognostic marker in PDAC. BMC Cancer 18:777
Haselmann V, Kurz A, Bertsch U, Hubner S, Olempska-Muller M, Fritsch J, Hasler R, Pickl A, Fritsche H, Annewanter F, Engler C, Fleig B, Bernt A, Roder C, Schmidt H, Gelhaus C, Hauser C, Egberts JH, Heneweer C, Rohde AM, Boger C, Knippschild U, Rocken C, Adam D, Walczak H, Schutze S, Janssen O, Wulczyn FG, Wajant H, Kalthoff H, Trauzold A (2014) Nuclear death receptor TRAIL-R2 inhibits maturation of let-7 and promotes proliferation of pancreatic and other tumor cells. Gastroenterology 146:278–290
Hawsawi O, Henderson V, Burton LJ, Dougan J, Nagappan P, Odero-Marah V (2018) High mobility group A2 (HMGA2) promotes EMT via MAPK pathway in prostate cancer. Biochem Biophys Res Commun 504:196–202
Heilmann T, Vondung F, Borzikowsky C, Kruger S, Elessawy M, Alkatout I, Wenners A, Bauer M, Klapper W, Rocken C, Maass N, Schem C, Trauzold A (2020) Cytoplasmic levels of high mobility group A2 determine survival prognoses in breast cancer patients. Int J Biol Mark 35:20–28
Hristov AC, Cope L, Reyes MD, Singh M, Iacobuzio-Donahue C, Maitra A, Resar LM (2009) HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol 22:43–49
Huang B, Yang J, Cheng Q, Xu P, Wang J, Zhang Z, Fan W, Wang P, Yu M (2018) Prognostic value of HMGA2 in human cancers: a meta-analysis based on literatures and TCGA datasets. Front Physiol 9:776
Jin C, Xue Y, Li Y, Bu H, Yu H, Zhang T, Zhang Z, Yan S, Lu N, Kong B (2018) A 2-protein signature predicting clinical outcome in high-grade serous ovarian cancer. Int J Gynecol Cancer 28:51–58
Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ (2005) RAS is regulated by the let-7 microRNA family. Cell 120:635–647
Kang R, Zhang Q, Zeh HJ 3rd, Lotze MT, Tang D (2013) HMGB1 in cancer: good, bad, or both? Clin Cancer Res 19:4046–4057
Kou B, Liu W, Tang X, Kou Q (2018) HMGA2 facilitates epithelial–mesenchymal transition in renal cell carcinoma by regulating the TGF-beta/Smad2 signaling pathway. Oncol Rep 39:101–108
Kumar MS, Armenteros-Monterroso E, East P, Chakravorty P, Matthews N, Winslow MM, Downward J (2014) HMGA2 functions as a competing endogenous RNA to promote lung cancer progression. Nature 505:212–217
Li K, Yang J, Chen J, Shi Y, Zhang Z, Chen W (2020) High mobility group AT-hook 2 and c-MYC as potential prognostic factors in pancreatic ductal adenocarcinoma. Oncol Lett 19:1584–1592
Mito JK, Agoston AT, Dal Cin P, Srivastava A (2017) Prevalence and significance of HMGA2 expression in oesophageal adenocarcinoma. Histopathology 71:909–917
Morishita A, Zaidi MR, Mitoro A, Sankarasharma D, Szabolcs M, Okada Y, D’Armiento J, Chada K (2013) HMGA2 is a driver of tumor metastasis. Cancer Res 73:4289–4299
Nie D, Zhang L, Guo Q, Mao X (2018) High mobility group protein A2 overexpression indicates poor prognosis for cancer patients: a meta-analysis. Oncotarget 9:1237–1247
Nissen MS, Langan TA, Reeves R (1991) Phosphorylation by cdc2 kinase modulates DNA binding activity of high mobility group I nonhistone chromatin protein. J Biol Chem 266:19945–19952
Piscuoglio S, Zlobec I, Pallante P, Sepe R, Esposito F, Zimmermann A, Diamantis I, Terracciano L, Fusco A, Karamitopoulou E (2012) HMGA1 and HMGA2 protein expression correlates with advanced tumour grade and lymph node metastasis in pancreatic adenocarcinoma. Histopathology 60:397–404
Rahman MM, Qian ZR, Wang EL, Sultana R, Kudo E, Nakasono M, Hayashi T, Kakiuchi S, Sano T (2009) Frequent overexpression of HMGA1 and 2 in gastroenteropancreatic neuroendocrine tumours and its relationship to let-7 downregulation. Br J Cancer 100:501–510
Reeves R, Langan TA, Nissen MS (1991) Phosphorylation of the DNA-binding domain of nonhistone high-mobility group I protein by cdc2 kinase: reduction of binding affinity. Proc Natl Acad Sci U S A 88:1671–1675
Sgarra R, Pegoraro S, Ros G, Penzo C, Chiefari E, Foti D, Brunetti A, Manfioletti G (2018) High Mobility Group A (HMGA) proteins: molecular instigators of breast cancer onset and progression. Biochim Biophys Acta Rev Cancer 1869:216–229
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70:7–30
Strell C, Norberg KJ, Mezheyeuski A, Schnittert J, Kuninty PR, Moro CF, Paulsson J, Schultz NA, Calatayud D, Löhr JM, Frings O, Verbeke CS, Heuchel RL, Prakash J, Johansen JS, Ostman A (2017) Stroma-regulated HMGA2 is an independent prognostic marker in PDAC and AAC. Br J Cancer 117:65–77
Tallini G, Vanni R, Manfioletti G, Kazmierczak B, Faa G, Pauwels P, Bullerdiek J, Giancotti V, Van Den Berghe H, Dal Cin P (2000) HMGI-C and HMGI(Y) immunoreactivity correlates with cytogenetic abnormalities in lipomas, pulmonary chondroid hamartomas, endometrial polyps, and uterine leiomyomas and is compatible with rearrangement of the HMGI-C and HMGI(Y) genes. Lab Investig 80:359–369
Thuault S, Tan EJ, Peinado H, Cano A, Heldin CH, Moustakas A (2008) HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. J Biol Chem 283:33437–33446
Wang X, Liu X, Li AY, Chen L, Lai L, Lin HH, Hu S, Yao L, Peng J, Loera S, Xue L, Zhou B, Zhou L, Zheng S, Chu P, Zhang S, Ann DK, Yen Y (2011) Overexpression of HMGA2 promotes metastasis and impacts survival of colorectal cancers. Clin Cancer Res 17:2570–2580
Watanabe S, Ueda Y, Akaboshi S, Hino Y, Sekita Y, Nakao M (2009) HMGA2 maintains oncogenic RAS-induced epithelial–mesenchymal transition in human pancreatic cancer cells. Am J Pathol 174:854–868
Wu J, Wei JJ (2013) HMGA2 and high-grade serous ovarian carcinoma. J Mol Med (berl) 91:1155–1165
Wu J, Zhang S, Shan J, Hu Z, Liu X, Chen L, Ren X, Yao L, Sheng H, Li L, Ann D, Yen Y, Wang J, Wang X (2016) Elevated HMGA2 expression is associated with cancer aggressiveness and predicts poor outcome in breast cancer. Cancer Lett 376:284–292
Xi YN, Xin XY, Ye HM (2014) Effects of HMGA2 on malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells. Asian Pac J Trop Med 7:289–292
Xie F, Ling L, van Dam H, Zhou F, Zhang L (2018) TGF-beta signaling in cancer metastasis. Acta Biochim Biophys Sin (shanghai) 50:121–132
Zha L, Zhang J, Tang W, Zhang N, He M, Guo Y, Wang Z (2013) HMGA2 elicits EMT by activating the Wnt/beta-catenin pathway in gastric cancer. Dig Dis Sci 58:724–733
Zhang M, Hu D, Wang S, Qin C (2016) Clinicopathologic significance of HMGA2 expression’s correlation with prognosis of esophageal squamous cell carcinoma after Ivor Lewis esophagectomy. Minerva Chir 71:239–244
Zhu J, Wang H, Xu S, Hao Y (2017) Clinicopathological and prognostic significance of HMGA2 overexpression in gastric cancer: a meta-analysis. Oncotarget 8:100478–100489
Acknowledgements
We acknowledge financial support by Land Schleswig-Holstein within the funding program “Open Access Publikationsfonds”.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JPG, CH and AW wrote the manuscript. JPG and CH prepared the figures and evaluated the data. FMS and CH performed the histological examination and where together with JPG and CRöder major contributor to the data analyses. CRöcken, TB and HK supported infrastructure and organizational issues. AT designed the study, analyzed the data, designed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All patients have agreed to participate in the study and have signed informed consent before collecting material. This study was approved by the local institutional review board of the Medical Faculty of the Christian-Albrechts-University of Kiel (A-110/99).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gundlach, JP., Hauser, C., Schlegel, F.M. et al. Prognostic significance of high mobility group A2 (HMGA2) in pancreatic ductal adenocarcinoma: malignant functions of cytoplasmic HMGA2 expression. J Cancer Res Clin Oncol 147, 3313–3324 (2021). https://doi.org/10.1007/s00432-021-03745-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-021-03745-w