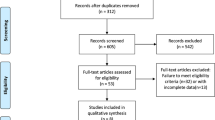
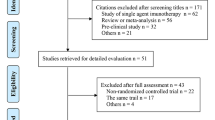
Abstract
Background
To evaluate the efficacy and safety of an immune checkpoint inhibitor (ICI) combined with chemotherapy in patients with advanced SCLC.
Methods
We searched published randomized-controlled trials (RCTs) to compare the effect of ICIs combined with chemotherapy and chemotherapy alone on SCLC. The extracted data included the number of people who achieved an objective response rate (ORR), the disease control rate (DCR), the hazard ratio (HR) of progression-free survival (PFS), and the overall survival (OS) with 95% confidence intervals (95% CI).
Results
Six RCTs involving 2477 patients were included. Compared with chemotherapy alone, patients receiving an ICI combined with chemotherapy had a significantly longer PFS (HR, 0.91; 95% CI 0.88–0.95, p < 0.00001) and OS (HR 0.92; 95% CI 0.89–0.96, p = 0.0001). The ORR increased, but the difference was not statistically significant (RR 1.05; 95% CI 0.99–1.12, p = 0.13). There was no significant difference in the DCR between the two treatment regimens; however, in patients treated with an ICI, fatigue, rashes, diarrhea, and elevated aminotransferase enzymes were significantly increased (p < 0.05).
Conclusion
ICI combined with chemotherapy is superior to chemotherapy alone with respect to PFS and OS in patients with advanced SCLC.







Similar content being viewed by others
Data availability
The data contained in this article can be applied.
References
Antonia SJ, Mirza N, Fricke I (2006) Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 12(3 Pt 1):878–887
Braly P, Nicodemus CF, Chu C et al (2009) The immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother 32(1):54–65
Bunn PJ, Minna JD, Augustyn A et al (2016) Small Cell lung cancer: can recent advances in biology and molecular biology be translated into improved outcomes? J Thorac Oncol 11(4):453–474
Chung HC, Lopez-Martin JA, Kao SC-H et al (2018) Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KeyNote-158. J Clin Oncol 36(15_suppl):8506
Curioni-Fontecedro A, Ickenberg C, Franzen D et al (2017) Diffuse pseudoprogression in a p-atient with metastatic non- small-cell lung cancer treated with Nivolumab. Ann Oncol 28(8):2040–2041
Gazdar AF, Bunn PA, Minna JD et al (2017) Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 17(12):725–737
Govindan R, Ding L, Griffith M et al (2012) Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 150(6):1121–1134
He Y, Rozeboom L, Rivard CJ et al (2017) MHC class II expression in lung cancer. Lung Cancer 112:75–80
Hellmann MD, Callahan MK, Awad MM et al (2018) Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 33(5):853–861
Hodi FS, O'Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723
Horn L, Mansfield AS, Szczesna A et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229
Kalemkerian GP, Schneider BJ (2017) Advances in small cell lung cancer. Hematol Oncol Clin North Am 31(1):143–156
Kashima J, Okuma Y, Shimizuguchi R et al (2018) Bile duct obstruction in a patient treated with nivolumab as second-line chemotherapy for advanced non-small-cell lung cancer: a case report. Cancer Immunol Immunother 67(1):61–65
Korkolopoulou P, Kaklamanis L, Pezzella F et al (1996) Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer 73(2):148–153
Krenacs T, Kiszner G, Stelkovics E et al (2012) Collagen XVII is expressed in malignant but not in benign melanocytic tumors and it can mediate antibody induced melanoma apoptosis. Histochem Cell Biol 138(4):653–667
List M, Jamous F, Gupta A et al (2015) Anti-Hu positive antibodies and small cell carcinoma: a single center review. S D Med 68(6):253–255
Liu G, Chen T, Li R et al (2018) Well-controlled pleural effusion indicated pseudoprogression after immunotherapy in lung cancer: a case report. Thorac Cancer 9(9):1190–1193
Melian M, Lorente D, Aparici F et al (2018) Lung brain metastasis pseudoprogression after nivolumab and ipilimumab combination treatment. Thorac Cancer 9(12):1770–1773
Naidoo J, Page DB, Li BT et al (2015) Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 26(12):2375–2391
Ott PA, Bang Y-J, Berton-Riguad D et al (2017) Safety and antitumor activity of pembrolizumab in advanced programmed death ligand-1-positive endometrial cancer: results from the K EY NOTE-028 study. J Clin Oncol 35(22):2535–2541
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Paz-Ares L, Dvorkin M, Chen Y et al (2019) Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 394(10212):1929–1939
Postow MA (2015) Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book 35:76–83
Reck M, Bondarenko I, Luft A et al (2013) Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 24(1):75–83
Reck M, Luft A, Szczesna A et al (2016) Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol 34(31):3740–3748
Ricciuti B, Kravets S, Dahlberg SE et al (2019) Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer 7(1):87
Rocha P, Hardy-Werbin M, Naranjo D et al (2018) CD103+CD8+ lymphocytes characterize the immune infiltration in a case with pseudoprogression in squamous NSCLC. J Thorac Oncol 13(10):e193–e196
Rudin CM, Awad MM, Navarro A et al (2020) Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, Phase III KEYNOTE-604 study. J Clin Oncol 38(21):2369–2379
Si X, Song P, Ni J et al (2020) Management of immune checkpoint inhibitor-related adverse events: a review of case reports. Thorac Cancer 11(3):498–504
Swami U, Smith M, Zhang J (2018) Central nervous system pseudoprogression with nivolumab in a patient with squamous cell lung cancer followed by prolonged response. J Thorac Oncol 13(9):e183–e184
Tazdait M, Mezquita L, Lahmar J et al (2018) Patterns of responses in metastatic NSCLC during PD-1 or PD-L1 inhibitor therapy: Comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 88:38–47
Travis WD, Brambilla E, Burke A et al (2015) Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol 10(9):1240–1242
Varghese AM, Zakowski MF, Yu HA et al (2014) Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 9(6):892–896
Wang Q, Gao J, Wu X (2018) Pseudoprogression and hyperprogression after checkpoint blockade. Int Immunopharmacol 58:125–135
Weber JS, Dummer R, de Pril V, Investigators MDX et al (2013) Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 119:1675–1682
Weber JS, Yang JC, Atkins MB et al (2015) Toxicities of immunotherapy for the practitioner. J Clin Oncol 33:2092–2099
Yamaura T, Suzuki H (2018) Pseudoprogression and rapid response to pembrolizumab in a patient with advanced lung adenocarcinoma with loss of epidermal growth factor receptor gene mutation after tyrosine kinase inhibitor therapy. J Thorac Oncol 13(10):e209–e210
Funding
This work was supported by National Natural Science Foundation of China (Grant No.81774221 and No.81904102), The Capital Health Research and Development of Special(No.2018-2-1113), Beijing Natural Science Foundation (No.7184203), Basic-Clinical Cooperation Program from Capital Medical University(No.17JL14), Research Foundation of Beijing Friendship Hospital, Capital Medical University (No.yyqdkt2016-4), and Beijing Municipal 215 High-level Health Person Foundation Project (No.2014-3-004).
Author information
Authors and Affiliations
Contributions
XTM and JY contributed to the conception and design this study. SW and YJZ carried out the development of the methodology. XTM and HMW analyzed and interpreted the data. JY and XTM wrote the manuscript and approved the final submission of the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, X., Wang, S., Zhang, Y. et al. Efficacy and safety of immune checkpoint inhibitors (ICIs) in extensive-stage small cell lung cancer (SCLC). J Cancer Res Clin Oncol 147, 593–606 (2021). https://doi.org/10.1007/s00432-020-03362-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03362-z