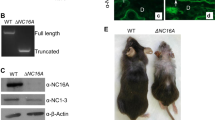
Abstract
The 180 kDa transmembrane collagen XVII is known to anchor undifferentiated keratinocytes to the basement membrane in hemidesmosomes while constitutively shedding a 120 kDa ectodomain. Inherited mutations or auto-antibodies targeting collagen XVII cause blistering skin disease. Collagen XVII is down-regulated in mature keratinocytes but re-expressed in skin cancer. By recently detecting collagen XVII in melanocyte hyperplasia, here we tested its expression in benign and malignant melanocytic tumors using endodomain and ectodomain selective antibodies. We found the full-length collagen XVII protein in proliferating tissue melanocytes, basal keratinocytes and squamous cell carcinoma whereas resting melanocytes were negative. Furthermore, the cell-residual 60 kDa endodomain was exclusively detected in 62/79 primary and 15/18 metastatic melanomas, 8/9 melanoma cell lines, HT199 metastatic melanoma xenografts and atypical nests in 8/63 dysplastic nevi. The rest of 19 nevi including common, blue and Spitz subtypes were also negative. In line with the defective ectodomain, sequencing of COL17A1 gene revealed aberrations in the ectodomain coding region including point mutations. Collagen XVII immunoreaction-stained spindle cell melanomas, showed partly overlapping profiles with those of S100B, Melan A and HMB45. It was concentrated at vertical melanoma fronts and statistically associated with invasive phenotype. Antibody targeting the extracellular aa507–529 terminus of collagen XVII endodomain promoted apoptosis and cell adhesion, while inhibiting proliferation in HT199 cells. These results suggest that the accumulation of collagen XVII endodomain in melanocytic tumors is associated with malignant transformation to be a potential marker of malignancy and a target for antibody-induced melanoma apoptosis.





Similar content being viewed by others
References
Alonso SR, Ortiz P, Pollan M, Perez-Gomez B, Sanchez L, Acuna MJ, Pajares R, Martinez-Tello FJ, Hortelano CM, Piris MA, Rodriguez-Peralto JL (2004) Progression in cutaneous malignant melanoma is associated with distinct expression profiles: a tissue microarray-based study. Am J Pathol 164:193–203
Balla P, Moskovszky L, Sapi Z, Forsyth R, Knowles H, Athanasou NA, Szendroi M, Kopper L, Rajnai H, Pinter F, Petak I, Benassi MS, Picci P, Conti A, Krenacs T (2011) Epidermal growth factor receptor signalling contributes to osteoblastic stromal cell proliferation, osteoclastogenesis and disease progression in giant cell tumour of bone. Histopathology 59:376–389
Bauer JW, Schaeppi H, Kaserer C, Hantich B, Hintner H (2001) Large melanocytic nevi in hereditary epidermolysis bullosa. J Am Acad Dermatol 44:577–584
Bichel J, Metze D, Bruckner-Tuderman L, Stander S (2001) Large melanocytic nevi in generalized atrophic benign epidermolysis bullosa (epidermolysis bullosa nevi). Hautarzt 52:812–816
Borradori L, Sonnenberg A (1999) Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol 112:411–418
Budinger L, Borradori L, Yee C, Eming R, Ferencik S, Grosse-Wilde H, Merk HF, Yancey K, Hertl M (1998) Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. J Clin Invest 102:2082–2089
Carlson JA, Ross JS, Slominski A, Linette G, Mysliborski J, Hill J, Mihm M Jr (2005) Molecular diagnostics in melanoma. J Am Acad Dermatol 52:743–775 (quiz 775–748)
Cohen-Knafo E, al Saati T, Aziza J, Ralfkiaer E, Selves J, Gorguet B, Delsol G (1995) Production and characterisation of an antimelanoma monoclonal antibody KBA.62 using a new melanoma cell line reactive on paraffin wax embedded sections. J Clin Pathol 48:826–831
Crowson AN, Magro CM, Mihm MC (2006) Prognosticators of melanoma, the melanoma report, and the sentinel lymph node. Mod Pathol 19(Suppl 2):S71–S87
de Vries TJ, Smeets M, de Graaf R, Hou-Jensen K, Brocker EB, Renard N, Eggermont AM, van Muijen GN, Ruiter DJ (2001) Expression of gp100, MART-1, tyrosinase, and S100 in paraffin-embedded primary melanomas and locoregional, lymph node, and visceral metastases: implications for diagnosis and immunotherapy. A study conducted by the EORTC Melanoma Cooperative Group. J Pathol 193:13–20
Franzke CW, Tasanen K, Borradori L, Huotari V, Bruckner-Tuderman L (2004) Shedding of collagen XVII/BP180: structural motifs influence cleavage from cell surface. J Biol Chem 279:24521–24529
Franzke CW, Bruckner P, Bruckner-Tuderman L (2005) Collagenous transmembrane proteins: recent insights into biology and pathology. J Biol Chem 280:4005–4008
Franzke CW, Bruckner-Tuderman L, Blobel CP (2009) Shedding of collagen XVII/BP180 in skin depends on both ADAM10 and ADAM9. J Biol Chem 284:23386–23396
Giblin AV, Thomas JM (2007) Incidence, mortality and survival in cutaneous melanoma. J Plast Reconstr Aesthet Surg 60:32–40
Giricz O, Lauer JL, Fields GB (2010) Variability in melanoma metalloproteinase expression profiling. J Biomol Tech 21:194–204
Gollob JA, Sciambi CJ, Huang Z, Dressman HK (2005) Gene expression changes and signaling events associated with the direct antimelanoma effect of IFN-gamma. Cancer Res 65:8869–8877
Ha L, Merlino G, Sviderskaya EV (2008) Melanomagenesis: overcoming the barrier of melanocyte senescence. Cell Cycle 7:1944–1948
Hofmann SC, Voith U, Schonau V, Sorokin L, Bruckner-Tuderman L, Franzke CW (2009) Plasmin plays a role in the in vitro generation of the linear IgA dermatosis antigen LADB97. J Invest Dermatol 129:1730–1739
Hopkinson SB, Findlay K, deHart GW, Jones JC (1998) Interaction of BP180 (type XVII collagen) and alpha6 integrin is necessary for stabilization of hemidesmosome structure. J Invest Dermatol 111:1015–1022
Huttenbach Y, Prieto VG, Reed JA (2002) Desmoplastic and spindle cell melanomas express protein markers of the neural crest but not of later committed stages of Schwann cell differentiation. J Cutan Pathol 29:562–568
Jangi SM, Ruiz-Larrea MB, Nicolau-Galmes F, Andollo N, Arroyo-Berdugo Y, Ortega-Martinez I, Diaz-Perez JL, Boyano MD (2008) Terfenadine-induced apoptosis in human melanoma cells is mediated through Ca2+ homeostasis modulation and tyrosine kinase activity, independently of H1 histamine receptors. Carcinogenesis 29:500–509
King R, Googe PB, Weilbaecher KN, Mihm MC Jr, Fisher DE (2001) Microphthalmia transcription factor expression in cutaneous benign, malignant melanocytic, and nonmelanocytic tumors. Am J Surg Pathol 25:51–57
Kitajima Y, Hirako Y, Owaribe K, Mori S, Yaoita H (1994) Antibody-binding to the 180-kD bullous pemphigoid antigens at the lateral cell surface causes their internalization and inhibits their assembly at the basal cell surface in cultured keratinocytes. J Dermatol 21:838–846
Krenacs T, Krenacs L, Raffeld M (2010) Multiple antigen immunostaining procedures. Methods Mol Biol 588:281–300
Ladanyi A, Timar J, Bocsi J, Tovari J, Lapis K (1995) Sex-dependent liver metastasis of human melanoma lines in SCID mice. Melanoma Res 5:83–86
Lanschuetzer CM, Emberger M, Hametner R, Klausegger A, Pohla-Gubo G, Hintner H, Bauer JW (2003) Pathogenic mechanisms in epidermolysis bullosa naevi. Acta Derm Venereol 83:332–337
Lin MS, Gharia MA, Swartz SJ, Diaz LA, Giudice GJ (1999) Identification and characterization of epitopes recognized by T lymphocytes and autoantibodies from patients with herpes gestationis. J Immunol 162:4991–4997
Lin MS, Fu CL, Giudice GJ, Olague-Marchan M, Lazaro AM, Stastny P, Diaz LA (2000) Epitopes targeted by bullous pemphigoid T lymphocytes and autoantibodies map to the same sites on the bullous pemphigoid 180 ectodomain. J Invest Dermatol 115:955–961
Magro CM, Crowson AN, Mihm MC (2006) Unusual variants of malignant melanoma. Mod Pathol 19(Suppl 2):S41–S70
Moore MW, Gasparini R (2011) FISH as an effective diagnostic tool for the management of challenging melanocytic lesions. Diagn Pathol 6:76
Nishie W, Lamer S, Schlosser A, Licarete E, Franzke CW, Hofmann SC, Jackow J, Sitaru C, Bruckner-Tuderman L (2010) Ectodomain shedding generates neoepitopes on collagen XVII, the major autoantigen for bullous pemphigoid. J Immunol 185:4938–4947
Nishie W, Kiritsi D, Nystrom A, Hofmann SC, Bruckner-Tuderman L (2011) Dynamic interactions of epidermal collagen XVII with the extracellular matrix: laminin 332 as a major binding partner. Am J Pathol 179:829–837
Ogawa H, Sakuma M, Morioka S, Kitamura K, Sasai Y, Imamura S, Inaba Y (1995) The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci 9:136–141
Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW (2008) Immunohistochemical characteristics of melanoma. J Cutan Pathol 35:433–444
Parikka M, Kainulainen T, Tasanen K, Vaananen A, Bruckner-Tuderman L, Salo T (2003) Alterations of collagen XVII expression during transformation of oral epithelium to dysplasia and carcinoma. J Histochem Cytochem 51:921–929
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Pinon P, Wehrle-Haller B (2011) Integrins: versatile receptors controlling melanocyte adhesion, migration and proliferation. Pigment Cell Melanoma Res 24:282–294
Qiao H, Shibaki A, Long HA, Wang G, Li Q, Nishie W, Abe R, Akiyama M, Shimizu H, McMillan JR (2009) Collagen XVII participates in keratinocyte adhesion to collagen IV, and in p38MAPK-dependent migration and cell signaling. J Invest Dermatol 129:2288–2295
Seppanen A, Autio-Harmainen H, Alafuzoff I, Sarkioja T, Veijola J, Hurskainen T, Bruckner-Tuderman L, Tasanen K, Majamaa K (2006) Collagen XVII is expressed in human CNS neurons. Matrix Biol 25:185–188
Seppanen A, Suuronen T, Hofmann SC, Majamaa K, Alafuzoff I (2007) Distribution of collagen XVII in the human brain. Brain Res 1158:50–56
Slominski A (2002) Coming of age of melanogenesis-related proteins. Arch Pathol Lab Med 126:775–777
Stelkovics E, Korom I, Marczinovits I, Molnar J, Rasky K, Raso E, Ficsor L, Molnar B, Kopper L, Krenacs T (2008) Collagen XVII/BP180 protein expression in squamous cell carcinoma of the skin detected with novel monoclonal antibodies in archived tissues using tissue microarrays and digital microscopy. Appl Immunohistochem Mol Morphol 16:433–441
Suzuki M, Murata S, Yaoita H, Nakagawa H (2002) An antibody to BP 180 kDa antigen is able to induce an increase of intracellular Ca2+ concentration in DJM-1 (human squamous cell carcinoma) cells. Autoimmunity 35:271–276
Szabad G, Kormos B, Pivarcsi A, Szell M, Kis K, Kenderessy Szabo A, Dobozy A, Kemeny L, Bata-Csorgo Z (2007) Human adult epidermal melanocytes cultured without chemical mitogens express the EGF receptor and respond to EGF. Arch Dermatol Res 299:191–200
Tasanen K, Floeth M, Schumann H, Bruckner-Tuderman L (2000) Hemizygosity for a glycine substitution in collagen XVII: unfolding and degradation of the ectodomain. J Invest Dermatol 115:207–212
Vergier B, Prochazkova-Carlotti M, de la Fouchardiere A, Cerroni L, Massi D, De Giorgi V, Bailly C, Wesselmann U, Karlseladze A, Avril MF, Jouary T, Merlio JP (2011) Fluorescence in situ hybridization, a diagnostic aid in ambiguous melanocytic tumors: European study of 113 cases. Mod Pathol 24:613–623
Yamada T, Endo R, Tsukagoshi K, Fujita S, Honda K, Kinoshita M, Hasebe T, Hirohashi S (1996) Aberrant expression of a hemidesmosomal protein, bullous pemphigoid antigen 2, in human squamous cell carcinoma. Lab Invest 75:589–600
Zone JJ, Taylor TB, Meyer LJ, Petersen MJ (1998) The 97 kDa linear IgA bullous disease antigen is identical to a portion of the extracellular domain of the 180 kDa bullous pemphigoid antigen, BPAg2. J Invest Dermatol 110:207–210
Acknowledgments
The authors are indebted to Jeno Ormos for inspiration, Janos Molnar for initiation of this work, Klara Rasky for contribution in antibody development, Ilona Marczinovics and Zsuzsanna Bata for help in antibody characterization, Laszlo Krenacs and Zsolt Orosz for sharing some of their facilities and melanoma cases and to Bela Molnar and 3DHistech staff, as well as Edit Parsch, Katalin Szekeres, Aniko Sarro, Violetta Piurko and Bernadett Kormos for excellent technical assistance. This work was supported by OTKA T62758 of Hungary.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Kiszner and E. Stelkovics equally contributed to this work.
Rights and permissions
About this article
Cite this article
Krenacs, T., Kiszner, G., Stelkovics, E. et al. Collagen XVII is expressed in malignant but not in benign melanocytic tumors and it can mediate antibody induced melanoma apoptosis. Histochem Cell Biol 138, 653–667 (2012). https://doi.org/10.1007/s00418-012-0981-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-012-0981-9