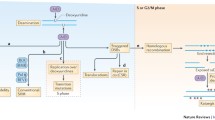
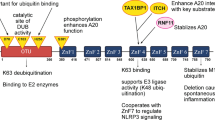
Abstract
Activation Induced cytidine Deaminase (AID) is an essential enzyme of the adaptive immune system. Its canonical activity is restricted to B lymphocytes, playing an essential role in the diversification of antibodies by enhancing specificity and changing affinity. This is possible through its DNA deaminase function, leading to mutations in DNA. In the last decade, AID has been assigned an additional function: that of a powerful DNA demethylator. Adverse cellular conditions such as chronic inflammation can lead to its deregulation and overexpression. It is an important driver of B-cell lymphoma due to its natural ability to modify DNA through deamination, leading to mutations and epigenetic changes. However, the deregulation of AID is not restricted to lymphoid cells. Recent findings have provided new insights into the role that this protein plays in the development of non-lymphoid cancers, with some research shedding light on novel AID-driven mechanisms of cellular transformation. In this review, we provide an updated narrative of the normal physiological functions of AID. Additionally, we review and discuss the recent research studies that have implicated AID in carcinogenesis in varying tissue types including lymphoid and non-lymphoid cancers. We review the mechanisms, whereby AID promotes carcinogenesis and highlight important areas of future research.

Similar content being viewed by others
Code availability
Not applicable.
Abbreviations
- A-EJ:
-
Alternative end-joining
- AID:
-
Activation induced cytidine deaminase
- AIDS:
-
Acquired immune deficiency disorder
- AOM:
-
Azoxymethane
- APE1:
-
Apyrimidinic endonuclease
- APOBEC:
-
Apolipoprotein B mRNA editing catalytic polypeptide-like
- ATP:
-
Adenosine triphosphate
- BER:
-
Base excision repair
- BL:
-
Burkitt lymphoma
- BUCC:
-
Bladder urothelial cell carcinoma
- CAC:
-
Colitis-associated cancers
- CHOP:
-
Cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate and prednisone
- CRM-1:
-
Chromosome maintenance 1
- CSR:
-
Class switch recombination
- dC:
-
Deoxycytidine
- DLBCL:
-
Diffuse large B cell lymphoma
- DSS:
-
Dextran sulfate sodium
- dU:
-
Deoxyuridine
- EBNA2:
-
Epstein-Barr virus nuclear antigen 2
- EBNA3C:
-
Epstein-Barr virus nuclear antigen 3C
- EBV:
-
Epstein-Barr virus
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial-to-mesenchymal transition
- GC:
-
Germinal centre
- HAART:
-
Highly active antiretroviral therapy
- HIV:
-
Human immunodeficiency virus
- IECs:
-
Intestinal epithelial cells
- IRC:
-
Inflammation-related carcinogenesis
- LMP1:
-
Latent Membrane Protein 1
- MMP14:
-
Matrix Metalloproteinase 14
- MMR:
-
Mismatch repair
- NES:
-
Nuclear export signal
- NHEJ:
-
Non-homologous end-joining
- NHL:
-
Non-Hodgkin lymphomas
- NLS:
-
Nuclear localisation signal
- PD-L1:
-
Programmed Cell Death Ligand
- RBPJ:
-
Recombination binding protein
- REG-γ:
-
REGgamma
- SHM:
-
Somatic hypermutation
- ssDNA:
-
Single stranded DNA
- TLR9:
-
Toll-like receptor 9
- TSS:
-
Transcription start site
- UNG:
-
Uracil-DNA glycosylase
- UV:
-
Ultraviolet
References
Aguilar R, Casabonne D, O’Callaghan-Gordo C, Vidal M, Campo JJ, Mutalima N et al (2017) Assessment of the combined effect of epstein-barr virus and plasmodium falciparum infections on endemic burkitt lymphoma using a multiplex serological approach. Front Immunol 8:1–11. https://doi.org/10.3389/fimmu.2017.01284
Álvarez-Prado ÁF, Pérez-Durán P, Pérez-García A, Benguria A, Torroja C, de Yébenes VG et al (2018) A broad atlas of somatic hypermutation allows prediction of activation-induced deaminase targets. J Exp Med 215:761–771. https://doi.org/10.1084/jem.20171738
Amir H, Mbonde MP, Kitinya JN (1992) Cutaneous squamous cell carcinoma in Tanzania. Cent Afr J Med 38:439–443
Anastasiadou E, Stroopinsky D, Alimperti S, Jiao AL, Pyzer AR, Cippitelli C et al (2019) Epstein-Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression dy downregulating miR-34a in B-cell lymphomas. Leukemia 33(1):132–147. https://doi.org/10.1038/s41375-018-0178-x
Araki A, Jin L, Nara H, Takeda Y, Nemoto N, Asao H et al (2019) IL-21 Enhances the development of colitis-associated colon cancer: possible involvement of activation-induced cytidine deaminase expression. J Immunol 18:ji1800550. https://doi.org/10.4049/jimmunol.1800550
Arima H, Fujimoto M, Nishikori M, Kondo T (2018) Prognostic impact of activation-induced cytidine deaminase expression for patients with diffuse large B-cell lymphoma. Leuk Lymphoma 59:2085–2095. https://doi.org/10.1080/10428194.2017.1410884
Basso K, Schneider C, Shen Q, Holmes AB, Setty M, Leslie C et al (2012) BCL6 positively regulates AID and germinal center gene expression via repression of miR-155. J Exp Med 209:2455–2465. https://doi.org/10.1084/jem.20121387
Bhutani N, Brady JJ, Damian M, Sacco A, Stéphane YB, Helen M (2010) Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature 463:1042–1047. https://doi.org/10.1038/nature08752.Reprogramming
Bi X-W, Wang H, Zhang W-W, Xia Z, Zhang Y-j et al (2016) PD-L1 is up-regulated by EBV-driven LMP1 through NF-kb pathway and correlates with poor prognosis in natural killer/T-Cell lymphoma. Lymphoma Biologu Non Gene studies. https://doi.org/10.1182/blood.V128.22.4134.4134
Bödör C, Bognár Á, Reiniger L, Szepesi Á, Tóth E, Kopper L et al (2005) Aberrant somatic hypermutation and expression of activation-induced cytidine deaminase mRNA in mediastinal large B-cell lymphoma. Br J Haematol 129:373–376. https://doi.org/10.1111/j.1365-2141.2005.05454.x
Borchert GM, Holton NW, Larson ED (2011) Repression of human activation induced cytidine deaminase by miR-93 and miR-155. BMC Cancer 11:347. https://doi.org/10.1186/1471-2407-11-347
Bornkamm GW (2009) Epstein-Barr virus and the pathogenesis of Burkitt’s lymphoma: More questions than answers. Int J Cancer 124:1745–1755. https://doi.org/10.1002/ijc.24223
Chandra V, Bortnick A, Murre C (2015) AID targeting: Old mysteries and new challenges. Trends Immunol 36:527–535. https://doi.org/10.1016/j.it.2015.07.003
Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA et al (2018) Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24:679–690. https://doi.org/10.1038/s41591-018-0016-8
Chaudhuri J, Evans T, Kumar R, DiMenna L (2014) Biological function of activation-induced cytidine deaminase (AID). Biomed J 37:269. https://doi.org/10.4103/2319-4170.128734
Chêne A, Donati D, Guerreiro-Cacais AO, Levitsky V, Chen Q, Falk KI et al (2007) A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog 3:0826–0834. https://doi.org/10.1371/journal.ppat.0030080
de Yébenes VG, Belver L, Pisano DG, González S, Villasante A, Croce C et al (2008) miR-181b negatively regulates activation-induced cytidine deaminase in B cells. J Exp Med 205:2199–2206. https://doi.org/10.1084/jem.20080579
Demorest ZL, Li M, Harris RS (2011) Phosphorylation directly regulates the intrinsic DNA cytidine deaminase activity of activation-induced deaminase and APOBEC3G protein. J Biol Chem 286:26568–26575. https://doi.org/10.1074/jbc.M111.235721
Dhokotera T, Bohlius J, Spoerri A, Egger M, Ncayiyana J, Olago V et al (2019) The burden of cancers associated with HIV in the South African public health sector, 2004–2014: a record linkage study. Infect Agent Cancer 14:12. https://doi.org/10.1186/s13027-019-0228-7
Dominguez PM, Shaknovich R (2014) Epigenetic function of activation-induced cytidine deaminase and its link to lymphomagenesis. Front Immunol 5:1–10. https://doi.org/10.3389/fimmu.2014.00642
Dominguez PM, Teater M, Chambwe N, Kormaksson M, Redmond D, Ishii J et al (2015) DNA methylation dynamics of germinal center B cells are mediated by AID. Cell Rep 12:2086–2098. https://doi.org/10.1016/j.celrep.2015.08.036
Dorsett Y, McBride KM, Jankovic M, Gazumyan A, Thai TH, Robbiani DF et al (2008) MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity 28:630–638. https://doi.org/10.1016/j.immuni.2008.04.002
Duquette ML, Pham P, Goodman MF, Maizels N (2005) AID binds to transcription-induced structures in c-MYC that map to regions associated with translocation and hypermutation. Oncogene 24:5791–5798. https://doi.org/10.1038/sj.onc.1208746
El-Amine R, Germini D, Zakharova VV, Tsfasman T, Sheval EV, Louzada RAN et al (2018) HIV-1 Tat protein induces DNA damage in human peripheral blood B-lymphocytes via mitochondrial ROS production. Redox Biol 15:97–108. https://doi.org/10.1016/j.redox.2017.11.024
Endo Y, Marusawa H, Kou T, Nakase H, Fujii S, Fujimori T et al (2008) Activation-induced cytidine deaminase links between inflammation and the development of colitis-associated colorectal cancers. Gastroenterology 135:889–898. https://doi.org/10.1053/j.gastro.2008.06.091
Epeldegui M, Breen EC, Hung YP, Boscardin WJ, Detels R, Martínez-Maza O (2007a) Elevated expression of activation induced cytidine deaminase in peripheral blood mononuclear cells precedes AIDS-NHL diagnosis. AIDS 21:2265–2270. https://doi.org/10.1097/QAD.0b013e3282ef9f59
Epeldegui M, Hung YP, McQuay A, Ambinder RF, Martínez-Maza O (2007b) Infection of human B cells with Epstein-Barr virus results in the expression of somatic hypermutation-inducing molecules and in the accrual of oncogene mutations. Mol Immunol 44:934–942. https://doi.org/10.1016/j.molimm.2006.03.018
Epeldegui M, Thapa DR, De La Cruz J, Kitchen S, Zack JA, Martínez-Maza O (2010) CD40 Ligand (CD154) Incorporated into HIV Virions induces activation-induced Cytidine Deaminase (AID) expression in human B lymphocytes. PLoS ONE 5:e11448. https://doi.org/10.1371/journal.pone.0011448
Epeldegui M, Conti DV, Guo Y, Cozen W, Penichet M, Martinez-Mara O (2019) Elevated numbers of PD-L1 expressing B cells are associated with the development of AIDS-NHL. Sci Rep 9:9371. https://doi.org/10.1038/s41598-019-45479-3
Epstein MA, Henle G, Achong BG, Barr YM (1965) Morphological and biological studies on a virus in cultured lymphoblasts from Burkitt’s lymphoma. J Exp Med 121:761–770. https://doi.org/10.1084/jem.121.5.761
Fear DJ (2013) Mechanisms regulating the targeting and activity of activation induced cytidine deaminase. Curr Opin Immunol 25:619–628. https://doi.org/10.1016/j.coi.2013.05.017
Gazumyan A, Timachova K, Yuen G, Siden E, Di Virgilio M, Woo EM et al (2011) Amino-terminal phosphorylation of activation-induced cytidine Deaminase suppresses c-myc/IgH translocation. Mol Cell Biol 31:442–449. https://doi.org/10.1128/mcb.00349-10
Germini D, Tsfasman T, Klibi M, El-Amine R, Pichugin A, Iarovaia OV et al (2017) HIV Tat induces a prolonged MYC relocalization next to IGH in circulating B-cells. Leukemia 31:2515–2522. https://doi.org/10.1038/leu.2017.106
Gloster HM, Neal K (2006) Skin cancer in skin of color. J Am Acad Dermatol 55:741–760. https://doi.org/10.1016/j.jaad.2005.08.063
Grande BM, Gerhard DS, Jiang A, Griner NB, Abramson JS, Alexander TB et al (2019) Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood 133:1313–1324. https://doi.org/10.1182/blood-2018-09-871418
Greisman HA, Lu Z, Tsai AG, Greiner TC, Yi HS, Lieber MR (2012) IgH partner breakpoint sequences provide evidence that AID initiates t(11;14) and t(8;14) chromosomal breaks in mantle cell and Burkitt lymphomas. Blood 120:2864–2867. https://doi.org/10.1182/blood-2012-02-412791
He B, Raab-Traub N, Casali P, Cerutti A (2003) EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to Induce T cell-independent Ig heavy chain class switching. J Immunol 171:5215–5224. https://doi.org/10.4049/jimmunol.171.10.5215
Heath E, Begue-Pastor N, Chaganti S, Croom-Carter D, Shannon-Lowe C, Kube D et al (2012) Epstein-Barr virus infection of naïve B cells in vitro frequently selects clones with mutated immunoglobulin genotypes: Implications for virus biology. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1002697
Hui KF, Yiu SPT, Tam KP, Chiang AKS (2019) Viral-targeted strategies against EBV-associated lymphoproliferative diseases. Front Oncol 9:1–18. https://doi.org/10.3389/fonc.2019.00081
Ito S, Nagaoka H, Shinkura R, Begum N, Nakata M, Honjo T et al (2003) Activation-induced Cytidine Deaminase shuttles between nucleus and Cytoplasm like Apolipoprotein B mRNA editing Catalytic polypeptide 1. PNAS 101:1975–1980
Jiao J, Jin Y, Zheng M, Zhang H, Yuan M, Lv Z et al (2019) AID and TET2 co-operation modulates FANCA expression by active demethylation in diffuse large B cell lymphoma. Clin Exp Immunol 195:190–201. https://doi.org/10.1111/cei.13227
Kalchschmidt JS, Bashford-Rogers R, Paschos K, Gillman ACT, Styles CT, Kellam P et al (2016) Epstein–Barr virus nuclear protein EBNA3C directly induces expression of AID and somatic mutations in B cells. J Exp Med 213:921–928. https://doi.org/10.1084/jem.20160120
Kasar S, Kim J, Improgo R, Tiao G, Polak P, Haradhvala N et al (2015) Whole-genome sequencing reveals activation-induced cytidine deaminase signatures during indolent chronic lymphocytic leukaemia evolution. Nat Commun 6:1–12. https://doi.org/10.1038/ncomms9866
Kawamura K, Wada A, Wang JY, Li Q, Ishii A, Tsujimura H et al (2015) Expression of activation-induced cytidine deaminase is associated with a poor prognosis of diffuse large B cell lymphoma patients treated with CHOP-based chemotherapy. J Cancer Res Clin Oncol. https://doi.org/10.1007/s00432-015-2001-7
Kim JH, Kim WS, Park C (2013) Epstein-Barr virus latent membrane protein 1 increases genomic instability through Egr-1-mediated up-regulation of activation-induced cytidine deaminase in B-cell lymphoma. Leuk Lymphoma 54:2035–2040. https://doi.org/10.3109/10428194.2013.769218
Kou T, Marusawa H, Kinoshita K, Endo Y, Okazaki IM, Ueda Y et al (2007) Expression of activation-induced cytidine deaminase in human hepatocytes during hepatocarcinogenesis. Int J Cancer 120:469–476. https://doi.org/10.1002/ijc.22292
Kretzmer H, Bernhart SH, Wang W, Haake AWM, Weniger MA, Bergmann AK et al (2015) DNA-methylome analysis in Burkitt and follicular lymphomas identifies differentially methylated regions linked to somatic mutation and transcriptional control. Nat Genet 47:1316–1325. https://doi.org/10.1038/ng.3413.DNA-methylome
Lee-Theilen M, Chaudhuri J (2010) Walking the AID tightrope. Nat Immunol 11:107–109. https://doi.org/10.1038/ni0210-107
Legason ID, Pfeiffer RM, Udquim KI, Bergen AW, Gouveia MH, Kirimunda S et al (2017) Evaluating the causal link between malaria infection and endemic Burkitt Lymphoma in Northern Uganda: a Mendelian randomization study. EBioMedicine 25:58–65. https://doi.org/10.1016/j.ebiom.2017.09.037
Li H, Li Q, Ma Z, Zhou Z, Fan J, Jin Y et al (2019) AID modulates carcinogenesis network via DNA demethylation in bladder urothelial cell carcinoma. Cell Death Dis 10:251. https://doi.org/10.1038/s41419-019-1472-x
Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, Kleinstein SH, Schatz DG (2008) Two levels of protection for the B cell genome during somatic hypermutation. Nature 451:841–845. https://doi.org/10.1038/nature06547
Lohr JG, Stojanov P, Lawrence MS, Auclair D, Chapuy B, Sougnez C et al (2012) Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A 109:3879–3884. https://doi.org/10.1073/pnas.1121343109
Luo Y, Liu Y, Wu L, Ma X, Liu Q, Huang F et al (2019) CUL7 E3 Ubiquitin Ligase mediates the degradation of activation-induced Cytidine Deaminase and regulates the Ig class switch recombination in B Lymphocytes. J Immunol 203:269–281. https://doi.org/10.4049/jimmunol.1900125
Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T et al (2007) Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med 13:470–476. https://doi.org/10.1038/nm1566
McBride KM, Barreto V, Ramiro AR, Stavropoulos P, Nussenzweig MC (2004) Somatic hypermutation is limited by CRM1-dependent nuclear export of activation-induced deaminase. J Exp Med 199:1235–1244. https://doi.org/10.1084/jem.20040373
McBride KM, Gazumyan A, Woo EM, Schwickert TA, Chait BT, Nussenzweig MC (2006) Regulation of hypermutation by activation-induced cytidine deaminase phosphorylation. Proc Natl Acad Sci 103:8798–8803. https://doi.org/10.1073/pnas.0603272103
Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G et al (2012) Burkitt’s lymphoma. Lancet 379:1234–1244. https://doi.org/10.1016/S0140-6736(11)61177-X
Morgan HD, Dean W, Coker HA, Reik W, Petersen-Mahrt SK (2004) Activation-induced cytidine deaminase deaminates 5-methylcytosine in DNA and is expressed in pluripotent tissues: Implications for epigenetic reprogramming. J Biol Chem 279:52353–52360. https://doi.org/10.1074/jbc.M407695200
Mu Y, Zelazowska MA, McBride KM (2017) Phosphorylation promotes activation-induced cytidine deaminase activity at the Myc oncogene. J Exp Med 214:3543–3552. https://doi.org/10.1084/jem.20170468
Munoz DP, Lee EL, Takayama S, Coppe JP, Heo SJ, Boffelli D et al (2013) Activation-induced cytidine deaminase (AID) is necessary for the epithelial-mesenchymal transition in mammary epithelial cells. Proc Natl Acad Sci 110:E2977–E2986. https://doi.org/10.1073/pnas.1301021110
Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T et al (2000) Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102:553–563. https://doi.org/10.1016/S0092-8674(00)00078-7
Nagaoka H, Tran TH, Kobayashi M, Aida M, Honjo T (2010) Preventing AID, a physiological mutator, from deleterious activation: Regulation of the genomic instability that is associated with antibody diversity. Int Immunol 22:227–235. https://doi.org/10.1093/intimm/dxq023
Nakamura M, Sugita K, Sawada Y, Yoshiki R, Hino R, Tokura Y (2011) High levels of activation-induced cytidine deaminase expression in adult T-cell leukaemia/lymphoma. Br J Dermatol 165:437–439. https://doi.org/10.1111/j.1365-2133.2011.10342.x
Neuberger MS, Harris RS, Di Noia J, Petersen-Mahrt SK (2003) Immunity through DNA deamination. Trends Biochem Sci 28:305–312. https://doi.org/10.1016/S0968-0004(03)00111-7
Nonaka T, Toda Y, Hiai H, Uemura M, Nakamura M, Yamamoto N et al (2016) Involvement of activation-induced cytidine deaminase in skin cancer development. J Clin Invest 126:1367–1382. https://doi.org/10.1172/JCI81522
Oakes CC, Martin-Subero JI (2018) Insight into origins, mechanisms, and utility of DNA methylation in B-cell malignancies. Blood 132:999–1006. https://doi.org/10.1182/blood-2018-02-692970
Orem J, Mbidde EK, Lambert B, De Sanjose S, Weiderpass E (2007) Burkitt’s lymphoma in Africa, a review of the epidemiology and etiology. Afr Health Sci 7:166–175. https://doi.org/10.5555/afhs.2007.7.3.166
Orthwein A, Patenaude AM, Affar EB, Lamarre A, Young JC, Di Noia JM (2010) Regulation of activation-induced deaminase stability and antibody gene diversification by Hsp90. J Exp Med 207:2751–2765. https://doi.org/10.1084/jem.20101321
Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RSK, Küppers R, Dalla-Favera R (2001) Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature 412:341–346. https://doi.org/10.1038/35085588
Pasqualucci L, Kitaura Y, Gu H, Dalla-Favera R (2006) From the cover: PKA-mediated phosphorylation regulates the function of activation-induced deaminase (AID) in B cells. Proc Natl Acad Sci 103:395–400. https://doi.org/10.1073/pnas.0509969103
Petersen-Mahrt SK, Harris RS, Neuberger MS (2002) AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. J Immunol 418:2043–2047. https://doi.org/10.1038/nature00849.1
Petrich AM, Sparano JA, Parekh S (2012) Paradigms and controversies in the treatment of HIV-related Burkitt lymphoma. Adv Hematol. https://doi.org/10.1155/2012/403648
Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M et al (2010) Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by Aid deficiency. Nature 463:1101–1105. https://doi.org/10.1038/nature08829.Genome-wide
Rai K, Huggins IJ, James SR, Karpf AR, Jones DA, Cairns BR (2008) DNA Demethylation in Zebrafish involves the coupling of a Deaminase, a Glycosylase, and Gadd45. Cell 135:1201–1212. https://doi.org/10.1016/j.cell.2008.11.042
Ramiro AR, Barreto VM (2015) Activation-induced cytidine deaminase and active cytidine demethylation. Trends Biochem Sci 40:172–181. https://doi.org/10.1016/j.tibs.2015.01.006
Ramiro AR, Jankovic M, Eisenreich T, Difilippantonio S, Chen-Kiang S, Muramatsu M et al (2004) AID is required for c-myc/IgH chromosome translocations in vivo. Cell 118:431–438. https://doi.org/10.1016/j.cell.2004.08.006
Rastelli J, Hömig-Hölzel C, Seagal J, Müller W, Hermann AC, Rajewskey K et al (2008) LMP1 signaling can replace CD40 signaling in B cells in vivo and has unique features of inducing class-switch recombination to IgG1. Blood 111:1448–1455. https://doi.org/10.1182/blood-2007-10-117655
Recaldin T, Hobson PS, Mann EH, Ramadani F (2018) miR-29b directly targets activation-induced cytidine deaminase in human B cells and can limit its inappropriate expression in naïve B cells. Mol Immunol 101:419–428. https://doi.org/10.1016/j.molimm.2018.07.028
Richter J, Schlesner M, Hoffmann S, Kreuz M, Leich E, Burkhardt B et al (2012) Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat Genet 44:1316–1320. https://doi.org/10.1038/ng.2469
Robbiani DF, Bothmer A, Callen E, Reina-San-Martin B, Dorsett Y, Difilippantonio S et al (2008) AID Is Required for the Chromosomal Breaks in c-myc that Lead to c-myc/IgH Translocations. Cell 135:1028–1038. https://doi.org/10.1016/j.cell.2008.09.062
Robbiani DF, Deroubaix S, Feldhahn N, Oliveira TY, Callen E, Wang Q et al (2015) Plasmodium infection promotes genomic Instability and AID-dependent B CELL LYMPHOMA. Cell 162:727–737. https://doi.org/10.1016/j.cell.2015.07.019
Rodrigo JA, Hicks LK, Cheung MC, Song KW, Ezzat H, Leger CS et al (2012) HIV-associated Burkitt lymphoma: Good efficacy and tolerance of intensive chemotherapy including CODOX-M/IVAC with or without rituximab in the HAART era. Adv Hematol. https://doi.org/10.1155/2012/735392
Sablitzky F, Wildner G, Rajewsky K (1985) Somatic mutation and clonal expansion of B cells in an antigen-driven immune response. EMBO J 4:345–350. https://doi.org/10.1002/j.1460-2075.1985.tb03635.x
Sall FB, El Amine R, Markozashvili D, Tsfasman T, Oksenhendler E, Lipinski M et al (2019) HIV-1 Tat protein induces aberrant activation of AICDA in human B-lymphocytes from peripheral blood. J Cell Physiol 234:15678–15685. https://doi.org/10.1002/jcp.28219
Sawai Y, Kodama Y, Shimizu T, Ota Y, Maruno T, Eso Y et al (2015) Activation-Induced Cytidine Deaminase contributes to pancreatic Tumorigenesis by inducing Tumor-related gene mutations. Cancer Res 75:3292–3301. https://doi.org/10.1158/0008-5472.CAN-14-3028
Takizawa M, Tolarová H, Li Z, Dubois W, Lim S, Callen E et al (2008) AID expression levels determine the extent of cMyc oncogenic translocations and the incidence of B cell tumor development. J Exp Med 205:1949–1957. https://doi.org/10.1084/jem.20081007
Talaei S, Mellatyar H, Asadi A, Akbarzadeh A, Sheervalilou R, Zarghami N (2019) Spotlight on 17-AAG as an Hso90 inhibitor for molecular targeted cancer treatment. Chem Biol Drug Des 93(5):760–786. https://doi.org/10.1111/cbdd.13486
Teater M, Dominguez PM, Redmond D, Chen Z, Ennishi D, Scott DW et al (2018) AICDA drives epigenetic heterogeneity and accelerates germinal center-derived lymphomagenesis. Nat Commun 3:1–10. https://doi.org/10.1038/s41467-017-02595-w
Teng G, Hakimpour P, Landgraf P, Rice A, Tuschl T, Casellas R et al (2008) MicroRNA-155 Is a negative regulator of activation-induced cytidine deaminase. Immunity 28:621–629. https://doi.org/10.1016/j.immuni.2008.03.015
Tobollik S, Meyer L, Buettner M, Klemmer S, Kempkes B, Kremmer E et al (2006) Epstein-Barr virus nuclear antigen 2 inhibits AID expression during EBV-driven B-cell growth. Blood 108:3859–3864. https://doi.org/10.1182/blood-2006-05-021303
Torgbor C, Awuah P, Deitsch K, Kalantari P, Duca KA, Thorley-Lawson DA (2014) A multifactorial role for P. falciparum Malaria in endemic Burkitt’s Lymphoma pathogenesis. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1004170
Tran TH, Nakata M, Suzuki K, Begum NA, Shinkura R, Fagarasan S et al (2010) B cell-specific and stimulation-responsive enhancers derepress Aicda by overcoming the effects of silencers. Nat Immunol 11:148–154. https://doi.org/10.1038/ni.1829
Uchimura Y, Barton LF, Rada C, Neuberger MS (2011) Reg-γ associates with and modulates the abundance of nuclear activation-induced deaminase. J Exp Med 208:2385–2391. https://doi.org/10.1084/jem.20110856
Wang Q, Kieffer-Kwon K-R, Oliveira TY, Mayer CT, Yao K, Pai J et al (2017) The cell cycle restricts activation-induced cytidine deaminase activity to early G1. J Exp Med 214:49–58. https://doi.org/10.1084/jem.20161649
Yokoyama S, Higashi M, Kitamoto S, Oeldorf M (2016) Aberrant methylation of MUC1 and MUC4 promoters are potential prognostic biomarkers for pancreatic ductal adenocarcinomas. Oncotarget 7:42553–42565
Yu K, Huang FT, Lieber MR (2004) DNA substrate length and surrounding sequence affect the activation-induced Deaminase activity at Cytidine. J Biol Chem 279:6496–6500. https://doi.org/10.1074/jbc.M311616200
Acknowledgements
Philipe Alves de Souza Rios for assisting in developing the graphics in Fig. 1.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
Not applicable.
Consent to participate:
Not applicable.
Consent for publication
All authors have consented to the publication of this manuscript.
Availability of data and material
Data sharing does not apply to this article as no datasets were generated or analysed during the current study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rios, L.A.d., Cloete, B. & Mowla, S. Activation-induced cytidine deaminase: in sickness and in health. J Cancer Res Clin Oncol 146, 2721–2730 (2020). https://doi.org/10.1007/s00432-020-03348-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-020-03348-x