Abstract
Purpose
Despite the advent of concomitant chemoradiotherapy (CCRT), the prognosis of advanced head and neck squamous cell carcinoma (HNSCC) patients remains particularly poor. Classically, HNSCC, especially oropharyngeal carcinomas, associated with human papillomavirus (HPV) exhibits better treatment outcomes than HNSCCs in non-infected patients, eliciting a call for the de-escalation of current therapies. To improve the management of HNSCC patients, we aimed to determine the impact of active HPV infection on patient response, recurrence and survival after CCRT in a population of heavy tobacco and alcohol consumers.
Methods
Paraffin-embedded samples from 218 advanced HNSCC patients, mostly smokers and/or drinkers treated by CCRT, were tested for the presence of HPV DNA by surrogate type-specific E6/E7 qPCR and p16 immunohistochemistry. Associations between the response to CCRT and patient outcomes according to HPV status and clinical data were evaluated by Kaplan–Meier analysis and both univariate and multivariate Cox regression.
Results
Type-specific E6/E7 PCR demonstrated HPV positivity in 20 % of HNSCC. Regarding HPV status, we did not find any significant relation with response to therapy in terms of progression-free survival or overall survival. However, we observed a significantly worse prognosis for consumers of alcohol and tobacco compared to nondrinkers (p = 0.003) and non-smokers (p = 0.03). Survival analyses also revealed that the outcome is compromised in stage IV patients (p = 0.007) and, in particular, for oral cavity, hypopharynx and oropharynx carcinoma patients (p = 0.001).
Conclusion
The risk of death from HNSCC significantly increases when patients are exposed to tobacco and alcohol during their therapy, regardless of HPV status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSCC) represents the fifth most common malignancy diagnosed worldwide. In 2012, HNSCCs accounted for approximately 59.000 new cases in the USA and more than 77.000 in Western and Eastern Europe (Globocan, 2012). These cancers form a group of heterogeneous tumors presenting distinct etiology, histology, risk factors and treatment approaches. Over the last twenty years, a clear increase in the incidence of oropharyngeal and oral cavity carcinomas has been observed, particularly in young adults in both the US and Europe, whereas the incidence of laryngeal carcinomas tended to remain stable or decrease slightly (D’Souza et al. 2007; Sturgis and Cinciripini 2007).
The advent of concomitant chemoradiotherapy (CCRT) in the early 2000s occurred in the phase III trial of Forastière et al’s study (Forastiere et al. 2003), who reported that the use of high-dose cisplatin and radiotherapy resulted in a considerable improvement in the survival of patients with laryngeal cancer. In addition, reduced mortality and improved locoregional control were observed upon treatment with both cetuximab and radiotherapy (Bonner et al. 2006). Currently, CCRT remains the gold standard to treat primary locally advanced head and neck cancer patients, especially those with stage III and IV disease (Forastiere et al. 2003; Bonner et al. 2006; Rosenthal et al. 2015). However, these aggressive treatments are characterized by tissue sequela (i.e., dry mucosa, muscle atrophy, and fibrosis leading to acute and chronic toxicities), morbidity (10 % of tracheotomy cases), and mortality (Trotti et al. 2003; Lazarus 2009; Hu et al. 2012; Hutcheson and Lewin 2012). It is therefore crucial to predict which patients will benefit from CCRT by investigating the impact of risk factors on the response to treatment.
Patients with HNSCCs often present a long history of tobacco and alcohol use. Recently, human papillomavirus (HPV) infection has emerged as an additional risk factor and could be involved in increased worldwide incidence of a subset of HNSCCs, especially oropharyngeal cancers (Fakhry and Gillison 2006; Sturgis and Cinciripini 2007; Fakhry et al. 2014). The development of cancers related to HPV infections has significantly complicated the profile of head and neck cancer patients, notably in terms of prognosis and response to treatment. The management of such patients is particularly complex in Europe, where many individuals are heavy smokers and/or drinkers (Duray et al. 2012; Duray et al. 2013). Indeed, while non-smoking and nondrinking oropharyngeal patients exhibit an improved response to therapy and a better outcome, tobacco and alcohol consumers with non-oropharyngeal cancers are associated with a heterogeneous prognosis (Ang et al. 2010; Isayeva et al. 2012). In this context, controversy exists regarding the prognosis of HPV+ patients treated by CCRT. Whereas several studies have reported that HPV infection is associated with a good prognosis (Kumar et al. 2007; Fakhry et al. 2008; Ang et al. 2010; Rischin et al. 2010; Hong et al. 2010; Nygård et al. 2012), other groups have reported opposing findings (Rosenquist et al. 2007; Lee et al. 2012; Duray et al. 2012). Thus, studies investigating the HPV status of HNSCC patients must be interpreted with caution because many are small clinical series without information regarding the alcohol consumption and smoking status of the patients.
The present study aims to determine the influence of HPV status on the response to CCRT and to estimate the impact of HPV infection as well as tobacco and alcohol consumption on recurrence and survival in a retrospective and prospective analysis of 218 head and neck cancer patients.
Materials and methods
Study population and clinical data
Formalin-fixed, paraffin-embedded HNSCC specimens were obtained from 218 patients (173 males, 45 females) who underwent concomitant chemoradiotherapy at the Saint-Pieter Hospital (Brussels) and Epicura Hospital (Baudour). Patients treated by cisplatin or Erbitux concomitant with radiotherapy were included in this study, and more than 95 % of the patients were stage III or IV. The response to treatment was evaluated three months after the end of treatment based on a clinical examination (endoscopy) and imaging technique (CT scan or MRI). On the basis of their cigarette and alcohol exposure, participants were classified as current, former or non-smokers and nondrinkers. Smokers/drinkers were defined as patients who continue to smoke and/or drink during their treatment, while formers include patients who stopped their consumption at diagnosis or for years before. Non-smokers and nondrinkers are individuals who have never used tobacco or alcohol. The clinical data collected from this series of 218 HNSCC patients are detailed in Table 1. This prospective and retrospective study was approved by the Institutional Review Board (AK/09-09-47/3805, P2014/185, as/2319).
DNA extraction and real-time PCR amplification of HPV type-specific DNA
The formalin-fixed, paraffin-embedded tissue samples (n = 218) were sectioned (10 × 5 µm), deparaffinized, and digested with proteinase K by overnight incubation at 56 °C. DNA was purified using the QIAamp DNA Mini Kit (Qiagen, Benelux, Belgium) according to the manufacturer’s recommended protocol. All DNA extracts were tested for the presence of 18 different HPV genotypes using TaqMan-based real-time PCR, as described previously (Depuydt et al. 2006, 2007; Duray et al. 2013).
p16 immunohistochemistry
Each HPV-positive case was further immunohistochemically evaluated for p16 expression using the recommended mouse monoclonal antibody (CINtec p16, Ventana, Tucson, USA) (Sawicka et al. 2013) and an automated immunostaining protocol (Bond-Max, Leica Microsystems, Wetzlar, Germany). Immunohistochemistry was performed on 5-µm thick tissue sections in the Leica Bond-Max immunostainer: The sections were deparaffinized, submerged in epitope retrieval solution (pH 6) for 10 min, and incubated with CINtec p16 antibody for 30 min. Then, polymer detection was performed using Bond Polymer Refine Detection according to the manufacturer’s protocol (Leica, Wetzlar, Germany), and the slides were counterstained with hematoxylin and luxol fast blue. Tissue sections from cervix lesions were used as positive controls. p16 expression was deemed positive only when the staining was both nuclear and cytoplasmic and when over 70 % of tumor cells were stained (Smeets et al. 2007).
Statistical analysis
Independent groups of categorical data were compared using the Pearson Chi-square test. Progression-free survival (PFS) and overall survival (OS) data were measured in terms of months from the date of diagnosis until disease recurrence or death or until the date at which the patient was last known to be alive. Standard survival time analyses were performed using Kaplan–Meier curves. For comparing two (or more) curves, univariate analyses were performed using the Cox regression model to estimate hazard ratios (HR), 95 % confidence intervals (CI), and associated p values. p values <0.05 were considered statistically significant. Multivariate Cox regression models were used to analyze the independent contribution of the HPV status to survival time in presence of other covariates such as conventional risk factors (stage, tobacco, alcohol) and response to CCRT. All statistical analyses were performed using Statistica (Statsoft, Tusla, OK, USA) and SPSS 15.0 Inc. (Chicago, IL, USA).
Results
Clinical data related to response, recurrence and survival in HNSCC patients
The response to CCRT, recurrence, and survival has been correlated with the different clinical data (Table 1). In terms of response and recurrence, only nasopharyngeal carcinoma fared significantly better than other cancers (p = 0.003) (data not shown). Gender did not significantly impact survival, although women seemed to present a higher lifetime survival (p = 0.11) (Fig. 1a). As expected, stage II and III patients had a longer survival compared to stage IV patients (p = 0.007) (Fig. 1b), and survival was also significantly higher for patients who responded to CCRT compared to non-responders (p < 0.001) (Fig. 1c). We also demonstrated that patients with nasopharyngeal or laryngeal carcinomas had a significantly better outcome than patients with carcinoma of the oropharynx, hypopharynx, or oral cavity (p = 0.001) (Fig. 1d).
Overall survival observed by a gender, b stages, c response rate to CCRT, and d tumor location for patients with HNSCCs. Prognosis appeared to be better for women than men, but the difference was not significant (hazard ratio [HR], 1.46; 95 % CI 0.92–2.34; p 0.11). Patients with stages II and III tumors had a significantly longer OS than patients with stage IV tumors (HR 1.90; 95 % CI 1.20–3.02; p 0.007), and non-responders to CCRT had a shorter lifetime than responders (HR 2.77; 95 % CI 1.92–3.98; p < 0.001). Regarding tumor location, the OS was significantly better for the patients with laryngeal and nasopharyngeal tumors compared to the patients with oral cavity, hypopharyngeal and oropharyngeal tumors (HR 1.29; 95 % CI 1.11–1.50; p 0.001)
HPV status and relation with clinical data in HNSCC patients
The 218 patients treated by CCRT were genotyped via real-time PCR using primers for 18 different HPV types (Fig. 2). HPV-positive cases were next analyzed for p16 immunohistochemical expression to distinguish transcriptionally active infections (p16+) from non-active infections (p16−) (Fig. 2b). Among our 218 patients, we identified 17 patients (8 %) whose tumors were positive for high-risk HPV and for p16, whereas 26 patients (12 %) infected by HPV were p16-negative, corresponding to a latent HPV infection. Among the HPV+ population, 5 cases presented insufficient tissue quantity for p16 immunohistochemistry, and therefore, they were excluded from the analyses. Overall, 170 patients (80 %) presented HPV- tumors according to real-time PCR analysis (Fig. 2a).
Assessment of HPV status in HNSCC patients. a HPV evaluation from 218 tumor tissues determined by real-time PCR and p16 immunohistochemistry. Five samples could not be analyzed by immunohistochemistry due to insufficient material. Among the 213 remaining cases, 170 (80 %) were negative for HPV, 26 (12 %) were positive for HPV but negative for p16, and 17 (8 %) were positive for both infection and p16 expression. b Typical p16 immunohistochemical expression corresponding to a transcriptionally active infection (p16+) and a non-active infection where p16 is not expressed (p16−)
The HPV+/p16+ group was composed of more men than women ranging from 48–79 years of age and mostly presenting stage IV disease (3 stage III and 14 stage IV) (Table 1). HPV+/p16+ cancers are more likely to develop in the oropharynx compared to other localizations (p = 0.007). In this subgroup of patients, there was a clear predominance of smokers (n = 10, 59 %) compared to patients who are former smokers (n = 4, 23 %) or who did not consume tobacco (n = 3, 18 %). In addition, about half of the patients were drinkers (n = 8, 47 %), but the proportion of nondrinkers was also high (n = 7, 41 %). However, no significant relation was found between HPV status and clinical data, including gender, smoking status, alcohol status, TNM stage, and treatment (Table 1).
Relation between HPV status and response rate to CCRT in HNSCC patients
Using the Pearson’s Chi-square test, we investigated whether HPV positivity is related to the rate of response to CCRT. For the three groups of patients (HPV−, HPV+/p16− and HPV+/p16+), there was no significant difference in the rate of responders versus non-responders because the percentage of responders versus non-responders was only slightly higher in HPV− (55 vs. 45 %) and HPV+/p16+ patients (53 vs. 47 %) (p = 0.7) (Table 1).
Relation between HPV infection, recurrence and survival in HNSCC patients
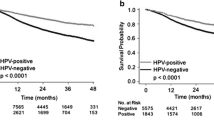
We did not observe any significant difference between the three populations of patients grouped by HPV status in terms of recurrence and survival (Fig. 3a, b). Indeed, at 5 years, the overall survival (OS) was slightly superior in the HPV+/p16+ subgroup, with 46 % of patients versus 38 and 40 % for both the HPV+/p16− and HPV− subgroups, respectively (Fig. 3b). However, this difference was not statistically significant (p > 0.05). Regarding Fig. 3a, the progression-free survival (PFS) at 5 years seemed to be slightly better for patients in the HPV+/p16+ subgroup compared to HPV+/p16− and HPV− patients, although the difference was not significant. We also evaluated the impact of a transcriptionally active infection on OS and compared the HPV+/p16+ patient group (active HPV) to a group combining HPV− and HPV+/p16− patients without finding any difference (Fig. 3c). PFS and OS were also evaluated in oropharyngeal carcinoma patients with respect to HPV status. The risk of death did not differ significantly between the HPV− and HPV+ patient groups, although we noted a trend to a better OS in active HPV patients, with a survival of 48 % at 5 years versus 31 % in the HPV− group (Fig. 3d), emphasizing the need to increase the HPV+/p16+ cohort to increase the statistical power. Moreover, we assessed the impact of HPV positivity on response and non-response to CCRT in patients affected by an oropharyngeal cancer, but we failed to demonstrate a significant relation between HPV infection and treatment response (Pearson’s Chi-square test, p = 0.4).
Evaluation of a PFS and b OS regarding HPV infection for patients with HNSCCs. Patients with HPV+/p16+ or HPV+/p16− tumors do not have a significant longer PFS (HR 1.12; 95 % CI 0.88–1.42; p 0.36) or OS (HR 1.14; 95 % CI 0.88–1.48; p 0.31) compared to HPV− patients. c Grouping patients according to transcriptionally active and non-active infection reveals no significant difference in OS (HR 1.01; 95 % CI 0.49–2.08; p 0.98) between the HPV+ group and the HPV− and HPV+/p16− groups. d Regarding the patients with oropharyngeal tumors, a trend to a better outcome was noted for the HPV+/p16+ patients, but the difference was not significant (HR 0.82; 95 % CI 0.35–1.92; p 0.64)
Smoking/drinking habits and HPV infection related to survival in HNSCC patients
Considering the high prevalence of smokers/former smokers and drinkers/former drinkers in our population, we evaluated whether the clinical patient outcome is compromised by smoking/drinking habits regardless of HPV status. We analyzed the impact on survival for non-smokers, former smokers and smokers separately and did not observe any significant differences. However, we also compared a group of non-smokers and former smokers against a group of smokers (who represent a large majority) and observed a significant association between smoking and a worse prognosis (p = 0.03) (Fig. 4a). Consistent with this finding, we similarly analyzed the impact of the alcohol intake status. This time, the grouping of former and current drinkers was successful to clearly exhibit significantly different outcomes as compared to nondrinkers. Indeed, in terms of OS, the rate of death due to cancer was significantly elevated in drinkers/former drinkers compared to nondrinkers (p = 0.003) (Fig. 4b).
Survival and tobacco/alcohol habits with respect to HPV status in HNSCC patients. a OS by smoking status. The prognosis for non- and former smokers was significantly better than for smokers (HR 1.49; 95 % CI 1.04–2.15; p 0.03). b OS by drinking status. The prognosis for nondrinkers was significantly better than for former and current drinkers (HR 2.11; 95 % CI 1.30–3.45; p 0.003). c Patient OS regarding HPV and smoking status. HPV status did not affect prognosis. *The HPV− non-smokers had a significantly longer OS than the HPV− smokers (HR 1.55; 95 % CI 1.06–2.27; p 0.025). d OS regarding HPV and drinking status. The analysis revealed a significant difference between the four survival curves (HR 1.34; 95 % CI 1.03–1.74; p 0.02). Moreover, *the HPV− nondrinkers had a greater chance of survival compared to the HPV− drinkers (HR 2.04; 95 % CI 1.22–3.43; p 0.007). e Fisher’s exact test illustrating the frequency of HPV+ and HPV− patients among smoker and non-smoker patients. No difference was observed in the proportion of active HPV+ patients among non-smokers or smokers (8 versus 8 % p = 1.0). f Fisher’s exact test illustrating the frequency of HPV+ and HPV− patients among drinker and nondrinker patients. The frequency of HPV+ patients was slightly higher among nondrinkers without statistical significance (14 versus 6 %, p 0.1)
We also analyzed the effect of the combination of active HPV infection and smoking status on OS (Fig. 4c). Globally, a comparison of 4 subgroups of patients did not reveal a significant difference between survival curves, but pairwise comparison demonstrated a significant difference in OS between HPV −/non-smokers and HPV −/smokers (*p = 0.025; HR 1.55; 95 % CI 1.06–2.27; Fig. 4c). The same comparison was carried out regarding drinking status, revealing a significant difference between the four curves (p = 0.02) (Fig. 4d). We proceeded to perform multiple pairwise comparisons and found that HPV− drinkers presented a poorer prognosis compared to HPV− nondrinkers (*p = 0.007; HR 2.04; 95 % CI 1.22–3.43; Fig. 4d). In this analysis, we also observed a tendency to a higher rate of death for active HPV+ drinker patients compared to HPV+ nondrinkers, but this association was not statistically significant because of the small group sizes (Fig. 4d). Finally, using the Fisher’s exact test, we examined the relation between HPV status and tobacco/alcohol status. The proportion of HPV+/p16+ patients was identical among non-smokers and smokers (Fig. 4e), whereas it was slightly higher among nondrinkers compared to drinkers. However, the latter difference was not significant (p = 0.1) (Fig. 4f).
Multivariate analysis of HPV status and impact on prognosis
Multivariate Cox regression models detailed in Table 2 show that the HPV status has no independent prognostic value with regard to conventional risk factors and therapy response (which presented significant survival impacts in univariate analyses). In contrast, Table 2 reports significant prognostic values for stage (II/III vs. IV), alcohol as well as response to CCRT with regard to both PFS and OS (with an additional significant contribution of the smoking status to OS).
Discussion
Locally advanced HPV+ HNSCCs represent a challenge for clinicians in terms of treatment strategy. This group of patients raises many therapeutic questions, including the choice of optimal treatment modality and the implications of HPV infection on the prognosis and response to CCRT. In our large population-based study, we demonstrated that the HPV status was neither associated with the response to CCRT nor the survival of HNSCC patients. We therefore reviewed previous studies examining HPV infection, response to CCRT and survival (Table 3). We noticed that very few studies have investigated correlations between such parameters and that they found a significant impact of HPV on the response to CCRT and an association with a better prognosis, unlike the findings reported in the current study (Kumar et al. 2007; Chung et al. 2009; Nichols et al. 2009; Fallai et al. 2009; de Jong et al. 2010; Ang et al. 2010; Rischin et al. 2010; Hong et al. 2010; Lill et al. 2011; Flavill et al. 2014; Hasegawa et al. 2014). This discrepancy with our findings can be explained by inclusion of smoker and/or drinker patients in our cohort and by the tumor location, which was not exclusively oropharyngeal. Moreover, smoking and drinking status was mostly imprecise or absent in previous studies, despite the fact that HPV+ tumors linked to tobacco and alcohol consumption represent a distinct biological and clinical entity. Indeed, Gillison and colleagues recently demonstrated that the outcome of treatment was compromised for p16+ and p16− patients who smoked during radiotherapy (Gillison et al. 2012). Unfortunately, HPV+ patients were rarely characterized according to the active nature of the infection, and even though an algorithm has been described that reliably identifies transcriptionally active HPV infection versus non-active infection in HNSCCs (Smeets et al. 2007). This distinction leads to a combination of p16 immunostaining followed by GP5 +/GP6 + PCR with 100 % specificity and sensibility. To our knowledge, ours is the first study to examine the implication of an active HPV infection in a large population of smoker/drinker HNSCCs and to reject the use of HPV as a predictive marker of response to treatment in this context.
Wide geographic variation has been reported regarding tobacco and alcohol consumption in Europe. Indeed, in western and eastern countries, the vast majority of patients are avid consumers, whereas a greater decline in smoking habits was observed among Norwegian, Finnish, and Dutch populations (Giskes et al. 2005; Tinhofer et al. 2015). In this context, there remains a lack of studies assessing tobacco and alcohol exposure in HPV-driven versus tobacco- and alcohol-associated HNSCCs. Thus, considering our smoker/drinker population, we tried to clarify the impact of HPV infection on patient prognosis as well as that of classical risk factors. The major findings of our population-based study are that smoking and drinking significantly increased the rate of death within 5 years after diagnosis in head and neck cancer patients, and that the prognostic behavior of former smokers is similar to that of non-smokers, while that of former drinkers remains relatively poor, such as current drinkers. Our statistic-based observations are fully supported by clinical data reporting that clinical benefits are rapidly observed following the cessation of tobacco, whereas the adverse effects of alcohol impact the health over a longer term and are less easily reversible (Doll et al. 2004). Studies conducted in consumer patients with HNSCCs have already demonstrated the negative impact of smoking tobacco and drinking alcohol on treatment response and OS. Twenty years ago, Browman et al. first reported that patients who continue to smoke during radiation therapy have lower rates of response and survival than patients who do not smoke during radiation therapy (Browman et al. 1993). These results were consistent across many studies that have found that smoking and drinking behavior can predict the clinical outcome of HNSCC patients (Dikshit et al. 2005; Park et al. 2006; Hilgert et al. 2009; Duffy et al. 2009; Chen et al. 2011; Hoff et al. 2012; Sharp et al. 2014). Indeed, through a large meta-analysis, Bagnardi et al. recently confirmed the higher risk of oral and pharyngeal cancer development for heavy drinkers compared to nondrinkers: Alcohol consumers have a 5.13 times higher relative risk of developing this type of tumor (Bagnardi et al. 2015).
The effect of tobacco use on disease recurrence was also examined among patients with HPV-positive oropharyngeal carcinomas. The typically good prognosis of HPV+ oropharyngeal carcinomas was not observed in our at-risk population. In fact, the HPV+ smoker group exhibited an increased risk of recurrence and distant metastases as well as reduced survival compared with the HPV+ non-smoker group (Maxwell et al. 2010). Many additional studies have found that HPV+ smokers exhibit reduced survival compared with HPV+ non-smokers, given the increased risk of both local recurrence and distant metastases in HPV+ smokers (Hafkamp et al. 2008; Kumar et al. 2008; Tribius et al. 2012; Lin et al. 2013).
Moreover, there is increasing support that HPV has developed several mechanisms to escape from immune surveillance and to maintain infection. Additionally, the tobacco use is known to suppress immune function, thereby facilitating persistent infection. Thus, the immunosuppressive mechanisms of smoking may prevent the patient from activating immunologic responses to eradicate the viral infection (Arnson et al. 2010). In this context, we speculate that there is an additive effect of smoking/drinking habits and HPV infection that leads to poorer outcomes in HNSCC patients, possibly due to DNA breaks resulting from tobacco usage in human cells during the process of HPV genome integration, which occurs at fragile sites or “hot spots” of DNA breakage. This mechanism thereby increases the carcinogenic potential of HPV (Hu et al. 2015). These observations suggest that smoking/drinking behavior and an immunosuppressive status promote HPV infection and persistence, leading to poor patient prognosis. These findings highlight the need to evaluate the role of tobacco and alcohol in the natural history of oral HPV infection and the progression to malignancy.
At this time, our data have demonstrated that active HPV infection cannot be used as a prognostic tool in non-oropharyngeal cancer patients. Our analysis is subject to limitations related to the low available number of HPV+/p16+ specimens as supported by a recent meta-analysis demonstrating that transcriptionally active infection rates are generally low for oral cavity and larynx cancer with 16.3 and 8.6 %, respectively (Gama et al. 2016). Nevertheless, our data clearly underscore that smoking and drinking during therapy significantly worsens patient prognosis and increases the risk of recurrence. As previously recommended (Gritz et al. 2005), all future clinical trials should measure tobacco and alcohol exposure to evaluate their effects on disease control alongside determining HPV status. Moreover, our data suggest that heavy tobacco and alcohol consumers who respond to CCRT should remain under close clinical and radiological follow-up at the end of treatment for the early detection of recurrences independent of HPV status and that clinicians should warn patients and encourage them to halt their consumption to better manage this high-risk subpopulation.
References
Fact Sheets by Population. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed 25 Mar 2016
Ang KK, Harris J, Wheeler R et al (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35. doi:10.1056/NEJMoa0912217
Arnson Y, Shoenfeld Y, Amital H (2010) Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 34:J258–J265. doi:10.1016/j.jaut.2009.12.003
Bagnardi V, Rota M, Botteri E et al (2015) Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 112:580–593. doi:10.1038/bjc.2014.579
Bonner JA, Harari PM, Giralt J et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578. doi:10.1056/NEJMoa053422
Browman GP, Wong G, Hodson I et al (1993) Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med 328:159–163. doi:10.1056/NEJM199301213280302
Chen AM, Chen LM, Vaughan A et al (2011) Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys 79:414–419. doi:10.1016/j.ijrobp.2009.10.050
Chung Y-L, Lee M-Y, Horng C-F et al (2009) Use of combined molecular biomarkers for prediction of clinical outcomes in locally advanced tonsillar cancers treated with chemoradiotherapy alone. Head Neck 31:9–20. doi:10.1002/hed.20913
D’Souza G, Kreimer AR, Viscidi R et al (2007) Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356:1944–1956. doi:10.1056/NEJMoa065497
de Jong MC, Pramana J, Knegjens JL et al (2010) HPV and high-risk gene expression profiles predict response to chemoradiotherapy in head and neck cancer, independent of clinical factors. Radiother Oncol J Eur Soc Ther Radiol Oncol 95:365–370. doi:10.1016/j.radonc.2010.02.001
Depuydt CE, Benoy IH, Bailleul EJ et al (2006) Improved endocervical sampling and HPV viral load detection by Cervex-Brush Combi. Cytopathol Off J Br Soc Clin Cytol 17:374–381. doi:10.1111/j.1365-2303.2006.00386.x
Depuydt CE, Boulet GV, Horvath CJ et al (2007) Comparison of MY09/11 consensus PCR and type-specific PCRs in the detection of oncogenic HPV types. J Cell Mol Med 11:881–891. doi:10.1111/j.1582-4934.2007.00073.x
Dikshit RP, Boffetta P, Bouchardy C et al (2005) Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: a multicentric European study. Int J Cancer 117:992–995. doi:10.1002/ijc.21244
Doll R, Peto R, Boreham J, Sutherland I (2004) Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 328:1519. doi:10.1136/bmj.38142.554479.AE
Duffy SA, Ronis DL, McLean S et al (2009) Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol 27:1969–1975. doi:10.1200/JCO.2008.18.2188
Duray A, Descamps G, Decaestecker C et al (2012) Human papillomavirus DNA strongly correlates with a poorer prognosis in oral cavity carcinoma. The Laryngoscope 122:1558–1565. doi:10.1002/lary.23298
Duray A, Descamps G, Decaestecker C et al (2013) Human papillomavirus predicts the outcome following concomitant chemoradiotherapy in patients with head and neck squamous cell carcinomas. Oncol Rep 30:371–376. doi:10.3892/or.2013.2415
Fakhry C, Gillison ML (2006) Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol Off J Am Soc Clin Oncol 24:2606–2611. doi:10.1200/JCO.2006.06.1291
Fakhry C, Westra WH, Li S et al (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100:261–269. doi:10.1093/jnci/djn011
Fakhry C, Zhang Q, Nguyen-Tan PF et al (2014) Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol Off J Am Soc Clin Oncol 32:3365–3373. doi:10.1200/JCO.2014.55.1937
Fallai C, Perrone F, Licitra L et al (2009) Oropharyngeal squamous cell carcinoma treated with radiotherapy or radiochemotherapy: prognostic role of TP53 and HPV status. Int J Radiat Oncol Biol Phys 75:1053–1059. doi:10.1016/j.ijrobp.2008.12.088
Flavill E, Fang YV, Miles B et al (2014) Induction chemotherapy followed by concurrent chemoradiotherapy for advanced stage oropharyngeal squamous cell carcinoma with HPV and P16 testing. Ann Otol Rhinol Laryngol 123:365–373. doi:10.1177/0003489414526685
Forastiere AA, Goepfert H, Maor M et al (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098. doi:10.1056/NEJMoa031317
Gama RR, Carvalho AL, Filho AL et al (2016) Detection of human papillomavirus in laryngeal squamous cell carcinoma: systematic review and meta-analysis. Laryngoscope 126:885–893. doi:10.1002/lary.25738
Gillison ML, Zhang Q, Jordan R et al (2012) Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol Off J Am Soc Clin Oncol 30:2102–2111. doi:10.1200/JCO.2011.38.4099
Giskes K, Kunst AE, Benach J et al (2005) Trends in smoking behaviour between 1985 and 2000 in nine European countries by education. J Epidemiol Community Health 59:395–401. doi:10.1136/jech.2004.025684
Gritz ER, Dresler C, Sarna L (2005) Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 14:2287–2293. doi:10.1158/1055-9965.EPI-05-0224
Hafkamp HC, Manni JJ, Haesevoets A et al (2008) Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer 122:2656–2664. doi:10.1002/ijc.23458
Hasegawa M, Maeda H, Deng Z et al (2014) Prediction of concurrent chemoradiotherapy outcome in advanced oropharyngeal cancer. Int J Oncol 45:1017–1026. doi:10.3892/ijo.2014.2504
Hilgert E, Bergmann C, Fichtner A et al (2009) Tobacco abuse relates to significantly reduced survival of patients with oropharyngeal carcinomas. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP 18:120–126. doi:10.1097/CEJ.0b013e32831012a4
Hoff CM, Grau C, Overgaard J (2012) Effect of smoking on oxygen delivery and outcome in patients treated with radiotherapy for head and neck squamous cell carcinoma–a prospective study. Radiother Oncol J Eur Soc Ther Radiol Oncol 103:38–44. doi:10.1016/j.radonc.2012.01.011
Hong AM, Dobbins TA, Lee CS et al (2010) Human papillomavirus predicts outcome in oropharyngeal cancer in patients treated primarily with surgery or radiation therapy. Br J Cancer 103:1510–1517. doi:10.1038/sj.bjc.6605944
Hu M, Ampil F, Clark C et al (2012) Comorbid predictors of poor response to chemoradiotherapy for laryngeal squamous cell carcinoma. The Laryngoscope 122:565–571. doi:10.1002/lary.22489
Hu Z, Zhu D, Wang W et al (2015) Genome-wide profiling of HPV integration in cervical cancer identifies clustered genomic hot spots and a potential microhomology-mediated integration mechanism. Nat Genet 47:158–163. doi:10.1038/ng.3178
Hutcheson KA, Lewin JS (2012) Functional outcomes after chemoradiotherapy of laryngeal and pharyngeal cancers. Curr Oncol Rep 14:158–165. doi:10.1007/s11912-012-0216-1
Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M (2012) Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol 6(Suppl 1):S104–S120. doi:10.1007/s12105-012-0368-1
Kumar B, Cordell KG, Lee JS et al (2007) Response to therapy and outcomes in oropharyngeal cancer are associated with biomarkers including human papillomavirus, epidermal growth factor receptor, gender, and smoking. Int J Radiat Oncol Biol Phys 69:S109–S111. doi:10.1016/j.ijrobp.2007.05.072
Kumar B, Cordell KG, Lee JS et al (2008) EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol Off J Am Soc Clin Oncol 26:3128–3137. doi:10.1200/JCO.2007.12.7662
Lazarus CL (2009) Effects of chemoradiotherapy on voice and swallowing. Curr Opin Otolaryngol Head Neck Surg 17:172–178. doi:10.1097/MOO.0b013e32832af12f
Lee L-A, Huang C-G, Liao C-T et al (2012) Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS ONE 7:e40767. doi:10.1371/journal.pone.0040767
Lill C, Kornek G, Bachtiary B et al (2011) Survival of patients with HPV-positive oropharyngeal cancer after radiochemotherapy is significantly enhanced. Wien Klin Wochenschr 123:215–221. doi:10.1007/s00508-011-1553-z
Lin BM, Wang H, D’Souza G et al (2013) Long-term prognosis and risk factors among patients with HPV-associated oropharyngeal squamous cell carcinoma. Cancer 119:3462–3471. doi:10.1002/cncr.28250
Maxwell JH, Kumar B, Feng FY et al (2010) Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res Off J Am Assoc Cancer Res 16:1226–1235. doi:10.1158/1078-0432.CCR-09-2350
Nichols AC, Faquin WC, Westra WH et al (2009) HPV-16 infection predicts treatment outcome in oropharyngeal squamous cell carcinoma. Otolaryngol-Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg 140:228–234. doi:10.1016/j.otohns.2008.11.025
Nygård M, Aagnes B, Bray F et al (2012) Population-based evidence of increased survival in human papillomavirus-related head and neck cancer. Eur J Cancer Oxf Engl 48:1341–1346. doi:10.1016/j.ejca.2012.03.014
Park SM, Lim MK, Shin SA, Yun YH (2006) Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol Off J Am Soc Clin Oncol 24:5017–5024. doi:10.1200/JCO.2006.07.0243
Rischin D, Young RJ, Fisher R et al (2010) Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol Off J Am Soc Clin Oncol 28:4142–4148. doi:10.1200/JCO.2010.29.2904
Rosenquist K, Wennerberg J, Annertz K et al (2007) Recurrence in patients with oral and oropharyngeal squamous cell carcinoma: human papillomavirus and other risk factors. Acta Otolaryngol (Stockh) 127:980–987. doi:10.1080/00016480601110162
Rosenthal DI, Harari PM, Giralt J et al (2015) Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab. J Clin Oncol Off J Am Soc Clin Oncol. doi:10.1200/JCO.2015.62.5970
Sawicka M, Pawlikowski J, Wilson S et al (2013) The specificity and patterns of staining in human cells and tissues of p16INK4a antibodies demonstrate variant antigen binding. PLoS ONE 8:e53313. doi:10.1371/journal.pone.0053313
Sharp L, McDevitt J, Carsin A-E et al (2014) Smoking at diagnosis is an independent prognostic factor for cancer-specific survival in head and neck cancer: findings from a large, population-based study. Cancer Epidemiol Biomark Prev 23:2579–2590. doi:10.1158/1055-9965.EPI-14-0311
Smeets SJ, Hesselink AT, Speel E-JM et al (2007) A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 121:2465–2472. doi:10.1002/ijc.22980
Sturgis EM, Cinciripini PM (2007) Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer 110:1429–1435. doi:10.1002/cncr.22963
Tinhofer I, Jöhrens K, Keilholz U et al (2015) Contribution of human papilloma virus to the incidence of squamous cell carcinoma of the head and neck in a European population with high smoking prevalence. Eur J Cancer Oxf Engl 51:514–521. doi:10.1016/j.ejca.2014.12.018
Tribius S, Hoffmann AS, Bastrop S et al (2012) HPV status in patients with head and neck of carcinoma of unknown primary site: HPV, tobacco smoking, and outcome. Oral Oncol 48:1178–1184. doi:10.1016/j.oraloncology.2012.05.022
Trotti A, Bellm LA, Epstein JB et al (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol J Eur Soc Ther Radiol Oncol 66:253–262
Acknowledgments
G.D. and N.K. are Ph.D. supported by a grant from the Télévie (Belgian National Fund for Scientific Research). We also thank to Vésale foundation, the Digital Image Analysis in Pathology (DIAPATH) and the Center for Microscopy and Molecular Imaging (CMMI, Gosselies, Belgium, supported by the European Regional Development Fund and the Walloon Region) for their contributions. C.D. is a senior research associate of the F.N.R.S. (Brussels, Belgium).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Descamps, G., Karaca, Y., Lechien, J.R. et al. Classical risk factors, but not HPV status, predict survival after chemoradiotherapy in advanced head and neck cancer patients. J Cancer Res Clin Oncol 142, 2185–2196 (2016). https://doi.org/10.1007/s00432-016-2203-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2203-7